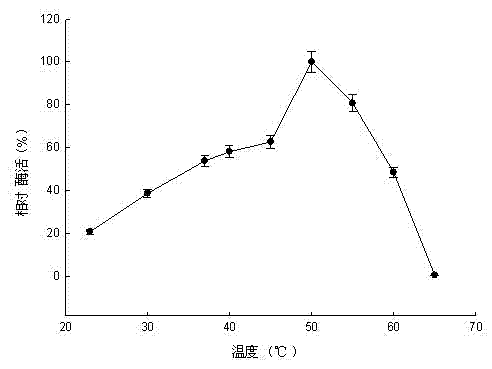
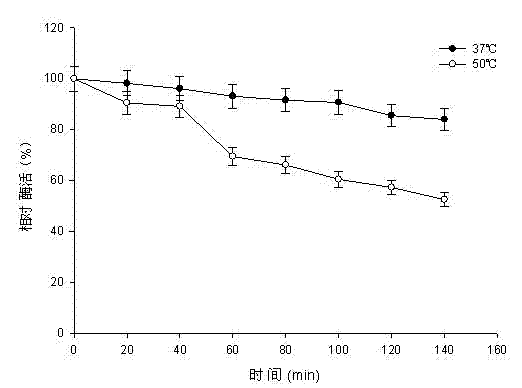
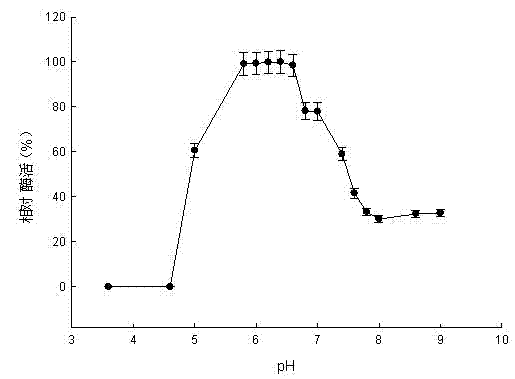
Pullulanase XWPu2 and gene thereof
A technology of pullulanase and gene, applied in the field of genetic engineering, can solve problems such as difficulty in mass production, low enzyme production of natural strains, and restrictions on popularization and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Pullulanase Gene wxya clone
[0034] Extraction of basophilic Paenibacillus ( Paenibacillussp.) Genomic DNA: CTAB lysis method, the specific steps are: centrifuge the fresh bacterial liquid cultured in liquid for 12 h, and add 800 μL solution Ⅰ (20 mM Tris, pH8.0, 2 mM Na 2 -EDTA, final concentration 20 mg / mL lysozyme), incubated at 37°C for 30 min, added 100 μL 10% SDS and mixed up and down, then added 10 μL 10 mg / mL proteinase K, incubated at 37°C for 30 min, added 150 μL μL 5 M NaCl and 150 μL 10% CTAB solution were mixed upside down, incubated at 65°C for 20 min, divided into 600 μL tubes, and then added 600 μL phenol / chloroform / isopropanol (volume ratio 25:24:1) For extraction, centrifuge at 12000 rpm for 10 min at 4 °C, take 300 μL of the supernatant and extract again in 300 μL chloroform / isopropanol (volume ratio 24:1) to remove impurities, and centrifuge at 12000 rpm for 10 min at 4 °C , then take 200 μL of the supernatant and add 2 times the vol...
Embodiment 2
[0041] Embodiment 2: Preparation of recombinant pullulanase XWPu2
[0042] pET -XWPu2 of Escherichia coli BL21(DE3) strain and pET-only - Empty plasmid for 28a(+) Escherichia coli BL21(DE3) strain was inoculated in LB (50 μg / mL Kan) medium with 0.1% inoculum, and shaken rapidly at 37 °C for 16 h. Then inoculate the activated bacterial solution into fresh LB (50 μg / mL Kan) culture solution with 1% inoculum, and culture with rapid shaking for about 2–3 h (OD 600 After reaching 0.6–1.0), add IPTG at a final concentration of 0.7 mM for induction, and continue shaking culture at 20 °C for about 20 h or at 26 °C for about 8 h. Centrifuge at 12000 rpm for 5 min to collect the cells. After suspending the cells with an appropriate amount of pH7.0 Tris-HCl buffer solution, the cells were disrupted by ultrasonic waves in a low-temperature water bath. The above intracellular concentrated crude enzyme solution was centrifuged at 13,000 rpm for 10 min, the supernatant was aspirat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com