Electrolyte for driving of aluminum electrolytic capacitor and preparation method of main solute of electrolyte
A technology for aluminum electrolytic capacitors and electrolytes, applied in the direction of electrolytic capacitors, capacitors, chemical instruments and methods, etc., can solve problems such as low solubility, increased vapor pressure of electrolytes, and large product impedance, and achieve high and low temperature stability. The effect of prolonging the service life and resisting large ripple current
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
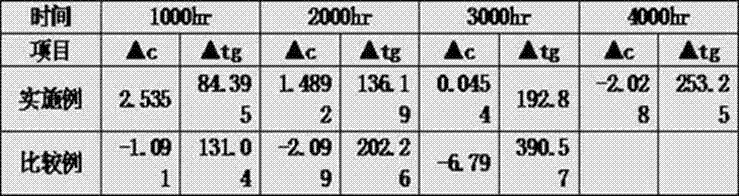
Image
Examples
Embodiment 1
[0031] Put 1000ml of absolute ethanol into a 2000ml three-necked flask, start stirring, and then slowly add 1mol of sodium metal. After all the sodium has reacted, cool to 30℃, add 1mol of ethyl 2-methylbutyrate, and stir for 40 Min, then raise the temperature to 50℃, slowly add 1 mol of ethyl 8-bromooctanoate (about 4h), slowly increase the temperature to 80℃ after dripping, reflux for 5h, sample and analyze, after all the raw materials have reacted, cool down To room temperature; filter and distill the mother liquor to remove anhydrous ethanol and unreacted raw materials. The temperature in the three-necked flask should not exceed 200°C, otherwise the product will be pumped out to obtain 2-methyl-2-ethyl sebacic acid. Ethyl; add 298g of diethyl 2-methyl-2-ethylsebacate to 4000ml solution of 1080g sodium hydroxide, heat to 100℃ and reflux for 12h; cool to room temperature, adjust PH=1 with phosphoric acid Before adjusting the pH, add 1000ml of distilled ethyl acetate; after th...
Embodiment 2
[0033] First put 1000ml of absolute ethanol into a 2000ml three-necked flask, start stirring, and then slowly add 1mol of sodium metal. After the sodium is completely reacted, cool to 40℃, add 1mol of ethyl 2-methylbutyrate, and stir for 30 Min, then raise the temperature to 60℃, slowly add 1 mol of ethyl 8-bromooctanoate (about 5h), slowly increase the temperature to 90℃ after dripping, reflux for 4h, sample and analyze, after all the raw materials have reacted, cool down To room temperature; filter and distill the mother liquor to remove anhydrous ethanol and unreacted raw materials. The temperature in the three-necked flask should not exceed 200°C, otherwise the product will also be pumped out to obtain 2-methyl-2-ethyl sebacic acid. Ethyl; add 271.89g of diethyl 2-methyl-2-ethylsebacate to 4000ml solution of 1080g sodium hydroxide, heat to 100℃ and reflux for 10h; cool to room temperature, adjust PH= 2. Before adjusting the pH, add 1000ml of distilled ethyl acetate; after t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com