Stereotactic fixing method of IgG antibody on surface of polystyrene carrier
A carrier surface, polystyrene technology, applied in the field of immunoassay, can solve the problems of losing binding antigen, inability to effectively expose antibody binding sites, unfavorable antibody-antigen binding, etc., and achieve the effect of improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Introduction of IDA-Ni on the surface of a polystyrene microwell plate 2+ active functional group
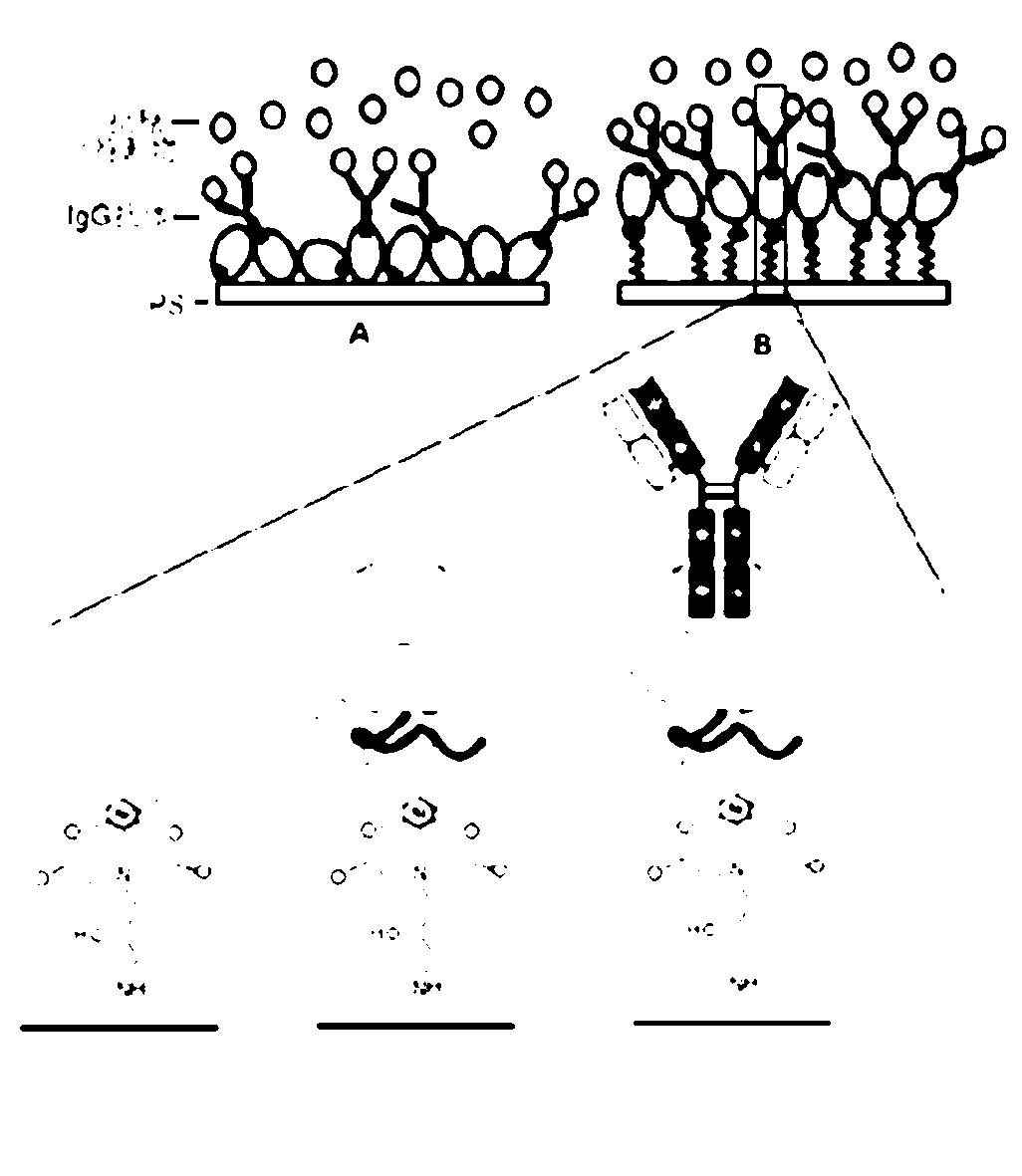
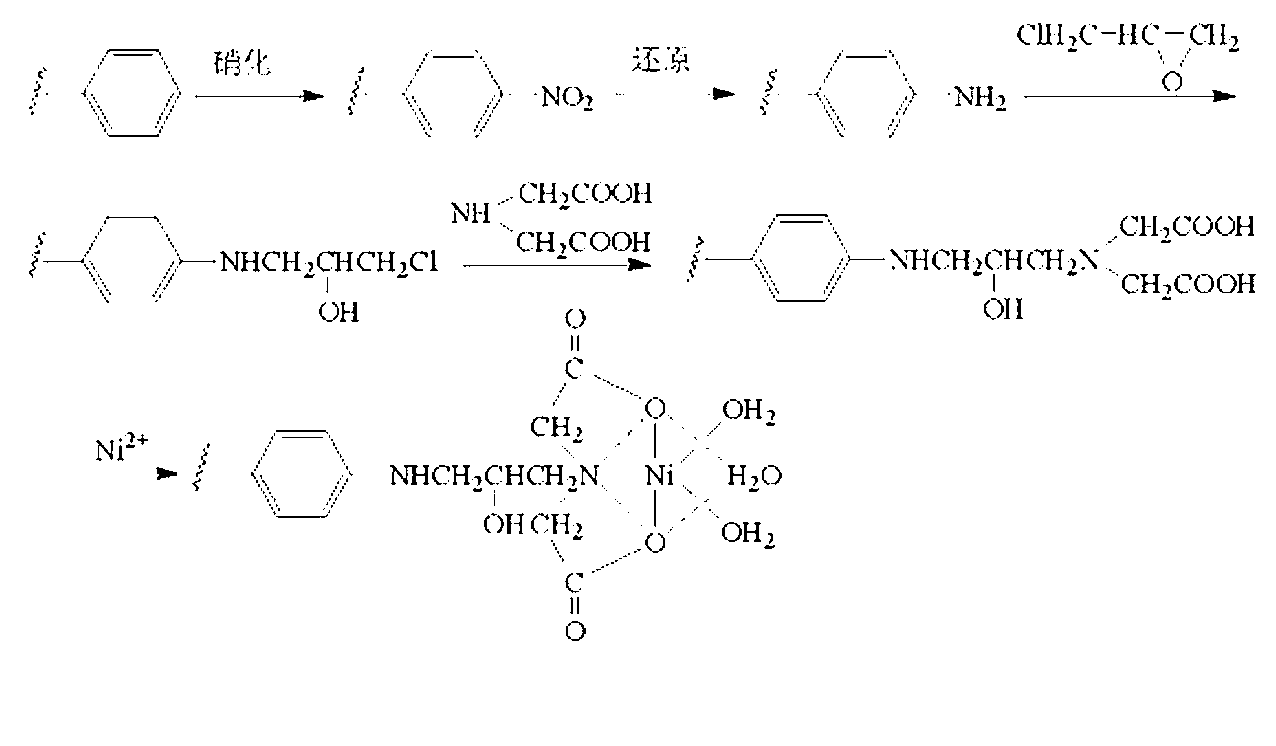
[0026] In order to bind the active Ni of His-tag on the surface of polystyrene microwell plate through metal affinity 2+ , first to activate the surface of polystyrene and introduce Ni that can chelate 2+ chemical groups such as figure 1 shown.
[0027] 1. Add 100 μL of 10% HNO to the microwells of the PS microplate 3 The glacial acetic acid solution was incubated at 60°C for 3 h, the reaction solution was removed, and the microplate was rinsed with deionized water.
[0028] 2. Add 100 μL containing 50% HNO to the microwell of PS microplate 3 Concentrated H 2 SO 4 The solution was kept at 60°C for 15 minutes, the reaction solution was removed, and the microplate was rinsed with deionized water.
[0029] 3. Add 100 μL of 1% Na 2 S 2 o 4 NaOH (0.1M) solution, kept at 60°C for 3h, removed the reaction solution, and rinsed the microplate with ...
Embodiment 2
[0039] Example 2, Preparation and Purification of ZZ-His Affinity Peptide by Genetic Engineering Technology
[0040] 1. Amplification of ZZ affinity peptide gene sequence, construction of vector and genetically engineered bacteria. Design primers: Primer 1: 5'-CCGGAATTCCATGGCGCAACACGATGAAG-3' and
[0041] Primer 2: 5'-CCCAAGCT TTTTATGATGATGATGATGATGATGATTCGCGTC-3'; using pEZZ18 as a template, the above primers PCR amplified the ZZ affinity peptide gene with His tag, cloned into pUC 18 vector to construct pUC-ZZ-His, and transformed E. coli DH5α, construct pUC-ZZ-His / E. coli DH5α engineering bacteria.
[0042] 2. Expression and purification of ZZ-His affinity peptide. pUC-ZZ-His / E. coli The DH5α engineered bacteria were activated overnight, transferred to fresh LB medium (ampicillin concentration 100 μg / mL) according to 5% inoculum, cultured with shaking at 37°C for 17-24 hours, and centrifuged to collect the bacteria. Resuspend the bacteria in PBS solution containi...
Embodiment 3
[0043] Embodiment three, PS-IDA-Ni 2+ Analysis of the directional immobilization ability of His tag protein
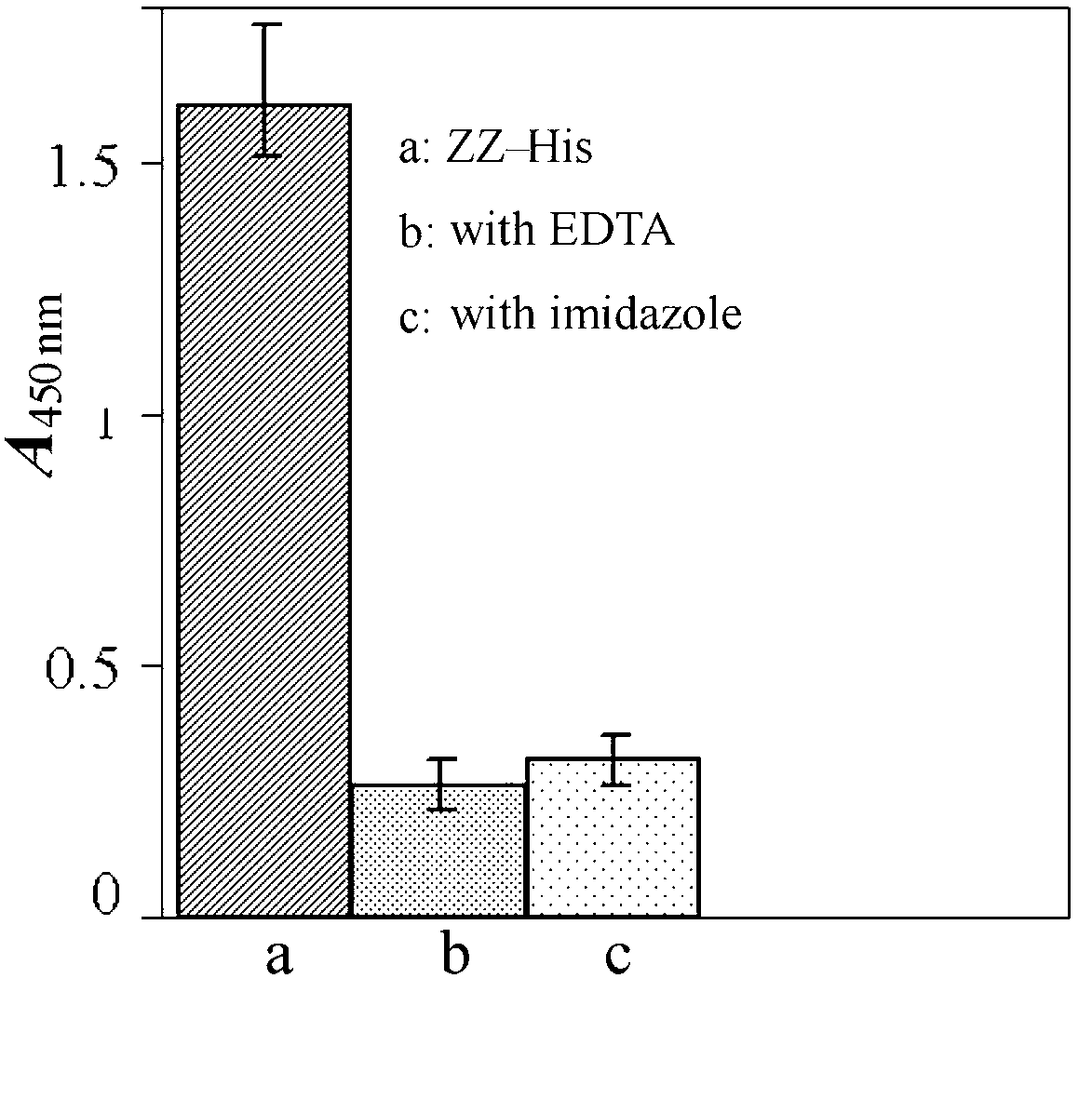
[0044] 1. Add 100 μL / well of ZZ-His (50 ng / mL, the solvent is PBS solution containing 20 mM imidazole, pH8.0) to PS–IDA–Ni 2+ Shake the microplate at room temperature for 30 minutes, remove the solution in the well, wash with PBST (containing 20nm imidazole) for 3 times, each time for 5 minutes, and dry it for later use.
[0045] 2. In order to determine whether the protein immobilized on the microwell plate is directional immobilized, that is, the binding ability of IgG antibody Fc segment, 100 μL / well rabbit anti-HRP antibody (200ng / mL) was added to ZZ-His / PS–IDA–Ni 2+ Microplate, incubated at 37°C for 1 hour, washed 3 times with PBST, added 100 μL / well of HRP (200ng / mL), incubated at 37°C for 1 hour, washed 3 times with PBST, HRP substrate 3,3' ,5,5'-Tetramethylbenzidine (TMB) for color development. The control experiment was the same concentration of ZZ-Hi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com