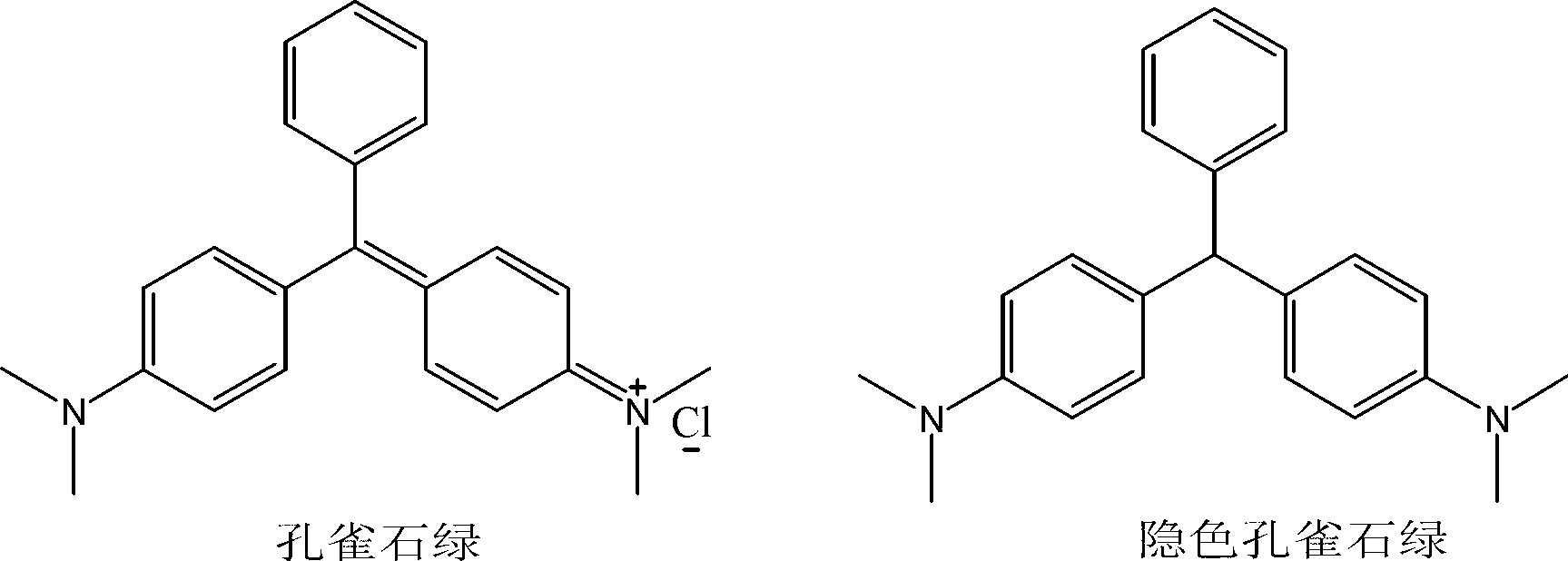
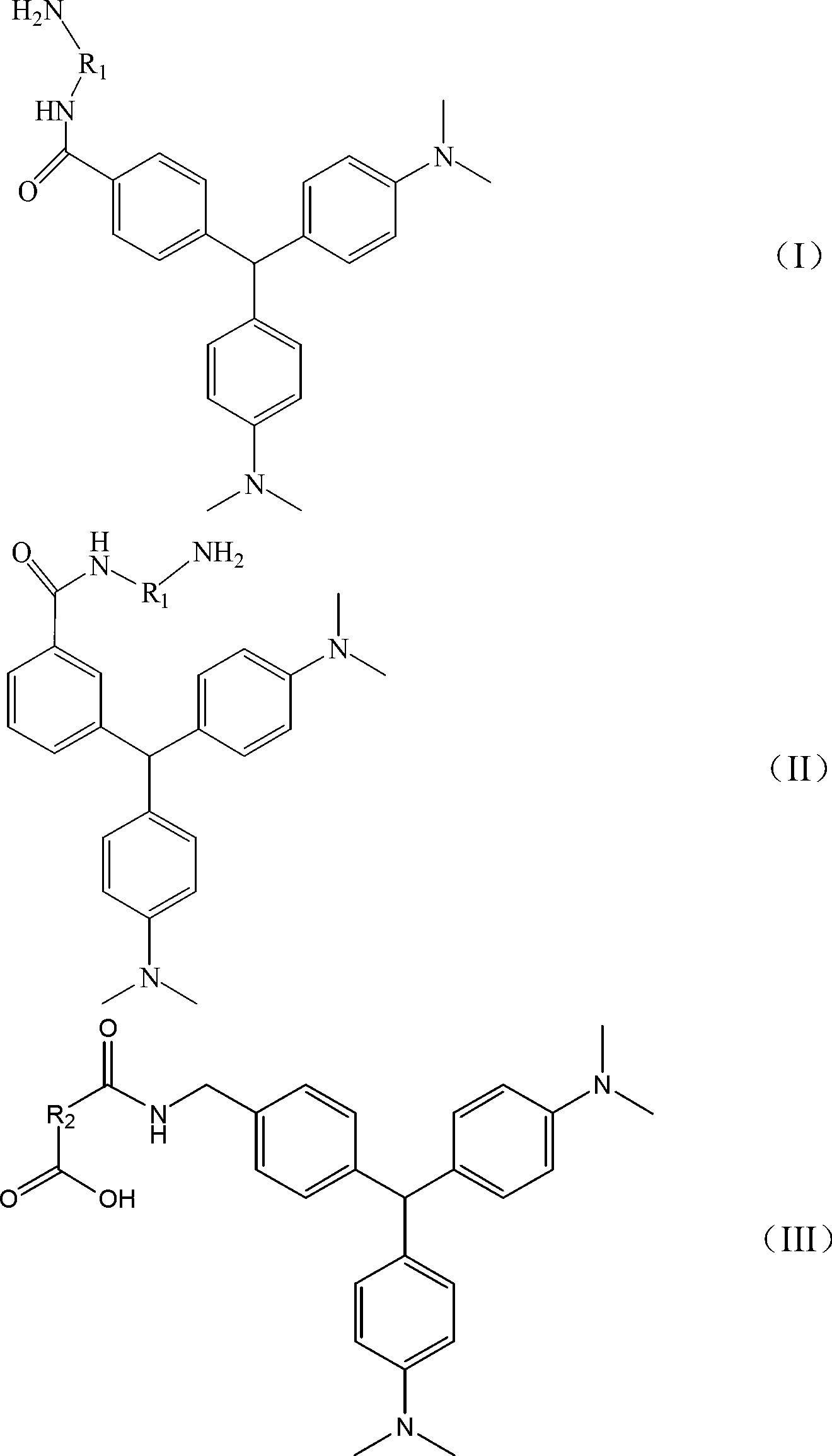
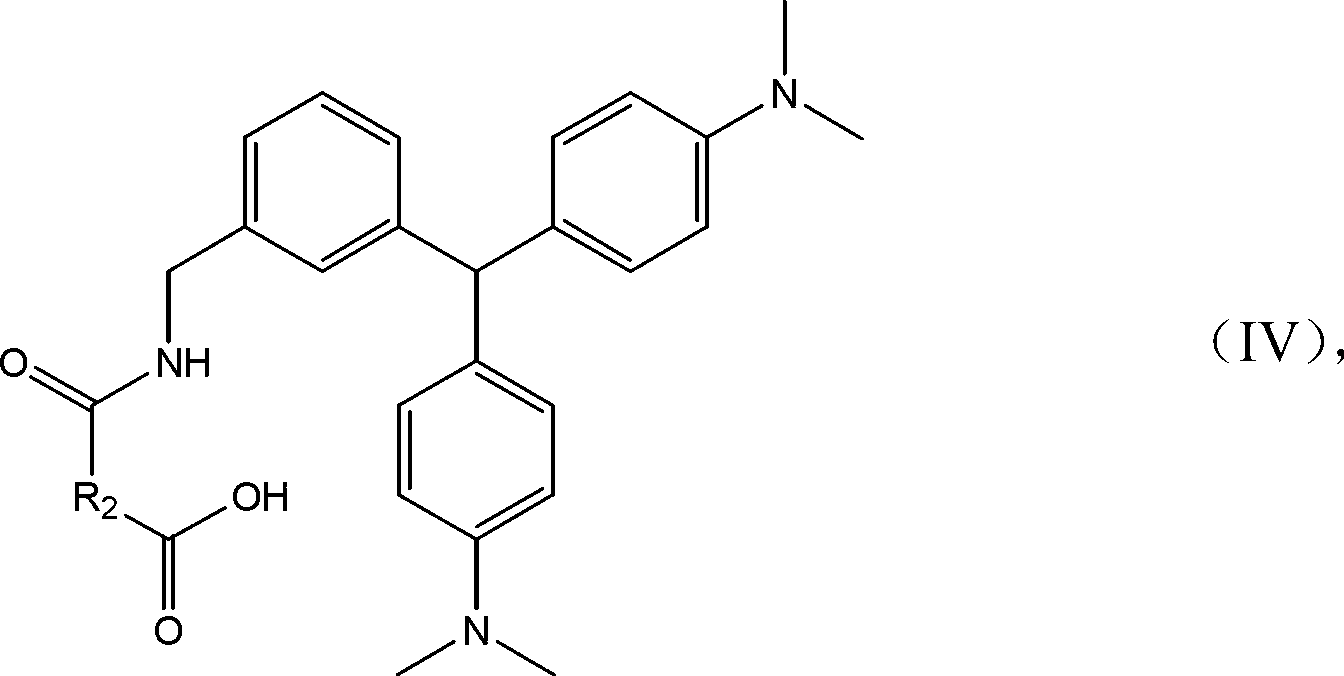
Malachite green hapten, malachite green artificial antigen and preparation method of malachite green hapten and artificial antigen
A leuco-malachite green, artificial antigen technology, applied in the field of immunochemistry, can solve the problems of weak antibody specificity and sensitivity, low recovery rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Take 2.5g (20mmol) of N,N-dimethylaniline into a 50mL two-necked flask, then slowly add 20mL of 5% Amberlyst15 resin toluene solution, stir magnetically for a few minutes, and add 1.50g p-formylbenzene under stirring Formic acid or m-formylbenzoic acid, stirred overnight at 80~100°C. After the reaction was completed, the catalyst was filtered, and saturated sodium bicarbonate solution was added to the reaction system to adjust the pH to 9.0, and extracted with dichloromethane. The pH of the aqueous phase was adjusted to 2.0 with dilute hydrochloric acid, extracted with ethyl acetate, and the organic phase was spun to dryness to obtain a crude product. Then add 2g of HATU, 0.5g of ethylenediamine, 2ml of DIPEA, dissolve with 20mL of DMF, stir with magnetic force at 50°C for 2h, add water to quench and extract with ethyl acetate, spin the organic phase to dryness, and finally use vacuum silica gel column chromatography Separation and purification yielded the desired prod...
Embodiment 2
[0076] Take 2.5g (20mmol) of N,N-dimethylaniline into a 50mL two-necked flask, slowly add 20mL of 5% Amberlyst15 resin toluene solution, stir magnetically for a few minutes, and add 1.50g of the compound of formula (XI) under stirring (where R 6 for CONH 2 , R 7 for H, or R 6 for H, R 7 for CONH 2 ), and stirred overnight at 80~100°C. After the reaction was completed, the catalyst was filtered, and saturated sodium bicarbonate solution was added to the reaction system to adjust the pH to 9.0, extracted with ethyl acetate, and the organic phase was rotary evaporated and spin-dried to obtain a crude product. Then dissolve in 10mL THF, add 1.5g Lithium Aluminum Hydride, stir the reaction with magnetic force at 0°C for 2h, quench with ammonium chloride solution, adjust the pH to 9.0, extract with ethyl acetate, spin the organic phase to dryness, and finally The desired compound was separated and purified by silica gel column chromatography. Then dissolve it with 20 mL of DM...
Embodiment 3
[0081] Take 2.5g (20mmol) of N,N-dimethylaniline into a 50mL two-necked flask, then slowly add 20mL of 5% Amberlyst15 resin toluene solution, stir magnetically for a few minutes, and add 1.50g p-formylbenzene under stirring Formic acid or m-formylbenzoic acid, stirred overnight at 80~100°C. After the reaction was completed, the catalyst was filtered and saturated sodium bicarbonate solution was added to the reaction system to adjust the pH to 9.0, and extracted with dichloromethane. The pH of the aqueous phase was adjusted to 2.0 with dilute hydrochloric acid, extracted with ethyl acetate, and the organic phase was spun to dryness to obtain a crude product. Then add 2g of HATU, 2g of the compound of formula (g), 2mL of DIPEA, dissolve in 20mL of DMF, stir with magnetic force at 50°C for 2h, add water to quench and extract with ethyl acetate, spin the organic phase to dryness, and finally use decompression silica gel The desired product was obtained by separation and purificat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com