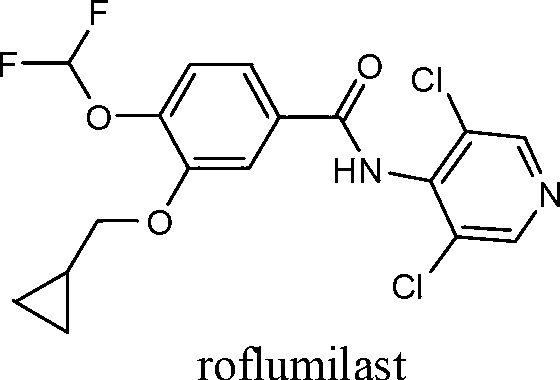
Method for synthesizing roflumilast
A technology of roflumilast and a synthesis method, which is applied to the synthesis field of roflumilast, can solve the problems of large-scale production danger, inconvenient scale-up production, low yield and the like, and achieves production safety, high purity and high purity. High yield, effect of reducing hygroscopicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
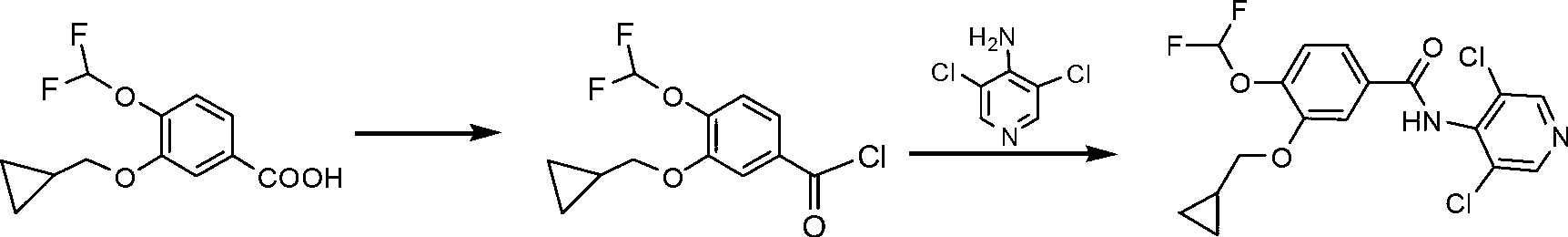
[0031] Embodiment 1 The synthetic method of roflumilast of the present invention
[0032] (1) Add 10.0g (about 0.039mol) 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid (compound Ⅱ), 16ml toluene, 1.0ml DMF (about 0.013mol) into the reaction flask, stir dissolve. Then add SOCl 2 3.6ml (about 0.05mol), heated to 80°C and stirred for 1h. Stop stirring and heating, and evaporate residual SOCl under reduced pressure after cooling slightly 2 and toluene; use 50ml of dry DMF to dissolve and set aside.
[0033] (2) Add 9.5g (about 0.058mol) of 4-amino-3,5-dichloropyridine (compound IV) and 50ml of dry DMF into the reaction flask, stir to dissolve. Add 3.0g (about 0.077mol) NaNH in batches under ice-water bath 2 After the addition, rinse the wall of the reaction flask with 10ml DMF, and stir for 40min. The DMF solution of compound III prepared in the first step was added dropwise into the reaction flask, and the reaction was stirred at room temperature for 5 h after the addit...
Embodiment 2
[0038] Embodiment 2 The synthetic method of roflumilast of the present invention
[0039] (1) Add 50.0g (about 0.19mol) 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid (compound Ⅱ), 75ml toluene, 2.0ml DMF (about 0.026mol) into the reaction flask, stir dissolve. Then add SOCl 2 18ml (about 0.248mol), heated to 70°C and stirred for 1h. Stop stirring and heating, and evaporate residual SOCl under reduced pressure after cooling slightly 2 and toluene; use 250ml of dry THF to dissolve and set aside.
[0040] (2) Add 47.4g (about 0.29mol) of 4-amino-3,5-dichloropyridine (compound IV) and 250ml of dry THF into the reaction flask, stir to dissolve. Add 19g NaNH in batches 2 (about 0.49mol), after adding, rinse the wall of the reaction flask with 50mlTHF. The reaction was stirred at room temperature for 40 min. The THF solution of compound III prepared in the first step was added dropwise into the reaction flask, and the reaction was stirred at room temperature for 5 h after t...
Embodiment 3
[0042] Embodiment 3 The synthetic method of roflumilast of the present invention
[0043] (1) Add 50.0g (about 0.19mol) of 3-cyclopropylmethoxy-4-difluoromethoxybenzoic acid (compound II), 75ml of toluene, and 0.114mol of DMF into the reaction flask, stir to dissolve. Then add 0.38molSOCl 2 , heated to 70 ° C and stirred for 1 h. Stop stirring and heating, and evaporate residual SOCl under reduced pressure after cooling slightly 2 and toluene; use 500ml of dry THF to dissolve and set aside.
[0044] (2) Add 52g (about 0.32mol) of 4-amino-3,5-dichloropyridine (compound IV) and 470ml of dry THF into the reaction flask, stir to dissolve. Add 25g NaNH in batches 2 (about 0.64mol), after adding, rinse the wall of the reaction flask with 50mlTHF. The reaction was stirred at room temperature for 40 min. The THF solution of compound III prepared in the first step was added dropwise into the reaction flask, and the reaction was stirred at room temperature for 5 h after the additi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com