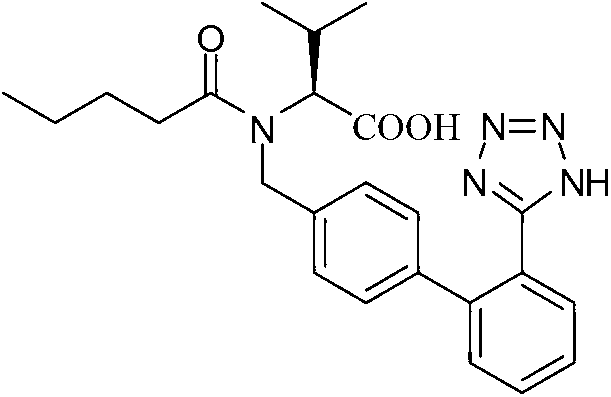
Novel method for preparing valsartan
A new method and technology of valsartan, applied in the field of preparation of antihypertensive drug valsartan, can solve the problems of low chemical purity and low optical purity of valsartan, and achieve simplified post-processing process, high product purity, and high yield. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
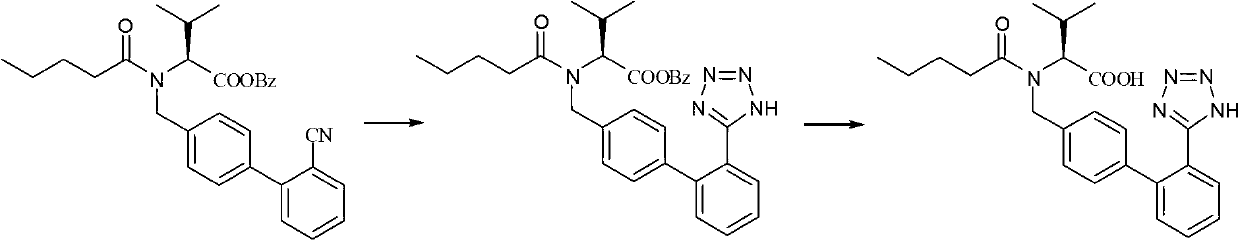
[0030] Embodiment 1: Preparation of the cyclization reaction solution of valsartan methyl ester.
[0031] Add 150ml toluene and 40.65g (0.1mol) N-(1-pentanoyl)-N-[4-[2-(5-cyano)phenyl]benzyl]-L-valline to a 500ml four-neck flask Acid methyl ester (1), stirring, heating up (about 30 ℃) to the compound (1-pentanoyl)-N-[4-[2-(5-cyano)phenyl]benzyl]-L-valline The acid methyl ester is completely dissolved. After the dissolution is complete, add 6.5g (0.1mol) sodium azide, 13.75g (0.1mol) triethylamine hydrochloride and 4g (0.03mol) zinc chloride to it, and heat up to 100°C Left and right, reflux reaction for 20 hours, HPLC tracking sample, after the reaction, start to drop the temperature, add 200ml water to it for washing, separate the lower water layer, wash the organic layer with 100ml saturated saline, separate the brine layer, and obtain the organic layer Be the toluene solution 160g of valsartan methyl ester crude product, contain valsartan methyl ester 22%.
Embodiment 2
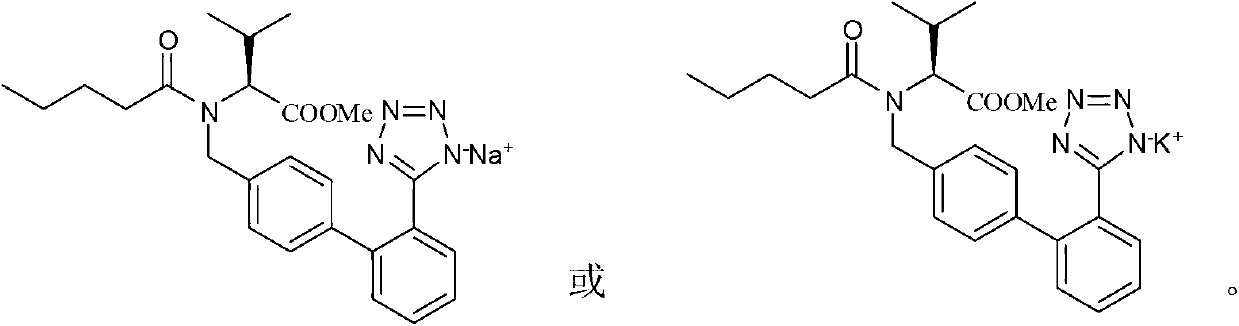
[0032] Embodiment 2: Preparation of valsartan methyl ester sodium salt.
[0033] In a 3000ml four-neck flask, add 1000g of valsartan methyl ester toluene solution (cyclization reaction solution prepared in Example 1, containing 22% of valsartan methyl ester), then add the solution made of 60g sodium bicarbonate and 540g water , stirred at room temperature to form a salt for 4 hours, filtered, washed with 100g of toluene, and then washed with 100g of water, and dried in vacuum at 80°C to constant weight to obtain 220g of white valsartan methyl ester sodium salt.
[0034] As detected by HPLC, the chemical purity is 99.4%.
[0035] Chiral HPLC detection, the optical purity is 99.2%.
[0036] 1 In HNMR analysis, there was no peak corresponding to the proton on the tetrazole ring.
[0037] Elemental analysis: C, 63.49%, H, 6.45%, N, 14.80%, Na, 4.85% (theoretical value: C, 63.68%, H, 6.41%, N, 14.85%, Na, 4.88%)
Embodiment 3
[0038] Embodiment 3: Preparation of valsartan methyl ester potassium salt.
[0039] Add 1000g valsartan methyl ester ethyl acetate solution (cyclization reaction solution, containing 25% valsartan methyl ester) in a 3000ml four-necked flask, then add a solution made of 80g sodium bicarbonate and 600g water, and stir at room temperature to form salt for 6 hours, filtered, washed with 100 g of toluene, and then washed with 100 g of water, and vacuum-dried at 80° C. to constant weight to obtain 260 g of white valsartan methyl ester potassium salt.
[0040] As detected by HPLC, the chemical purity is 99.5%.
[0041] Chiral HPLC detection, the optical purity is 99.1%.
[0042] 1 In HNMR analysis, there was no peak corresponding to the proton on the tetrazole ring.
[0043] Elemental analysis: C, 61.33%, H, 6.31%, N, 14.28%, K, 7.99% (theoretical value: C, 61.58%, H, 6.20%, N, 14.36%, K, 8.02%)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com