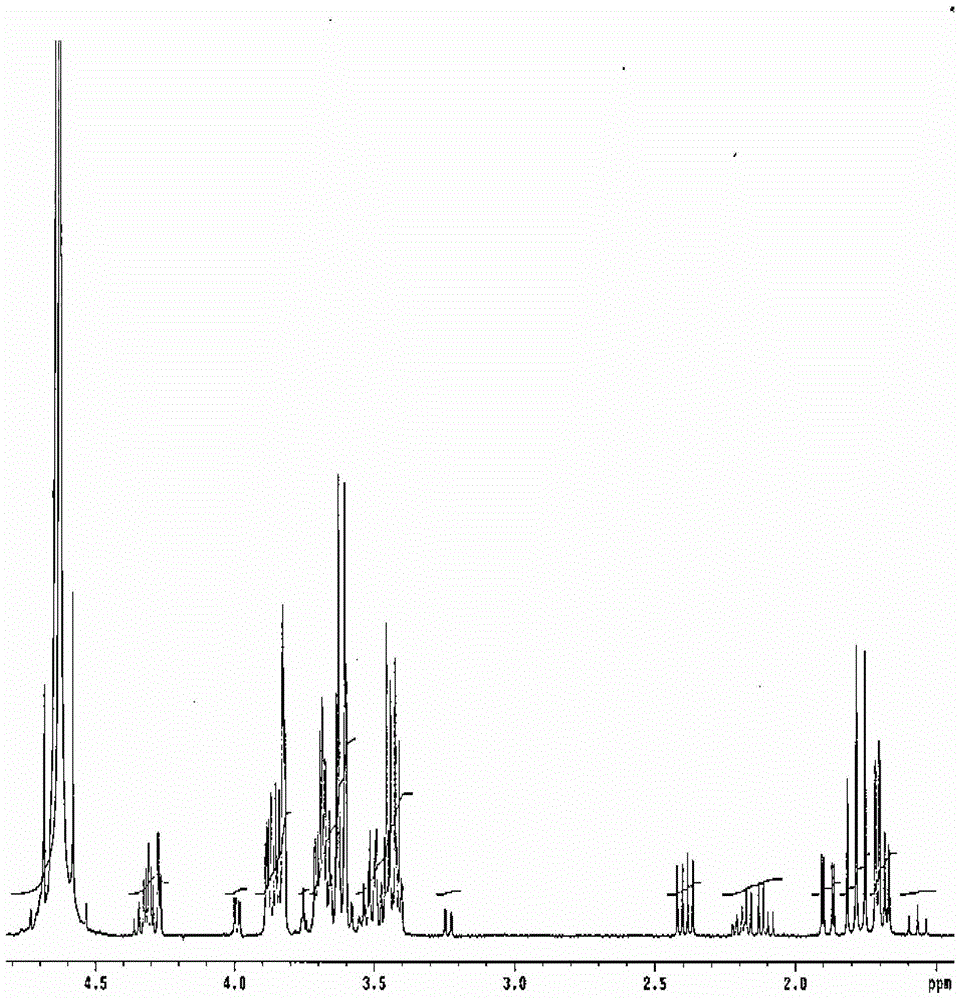
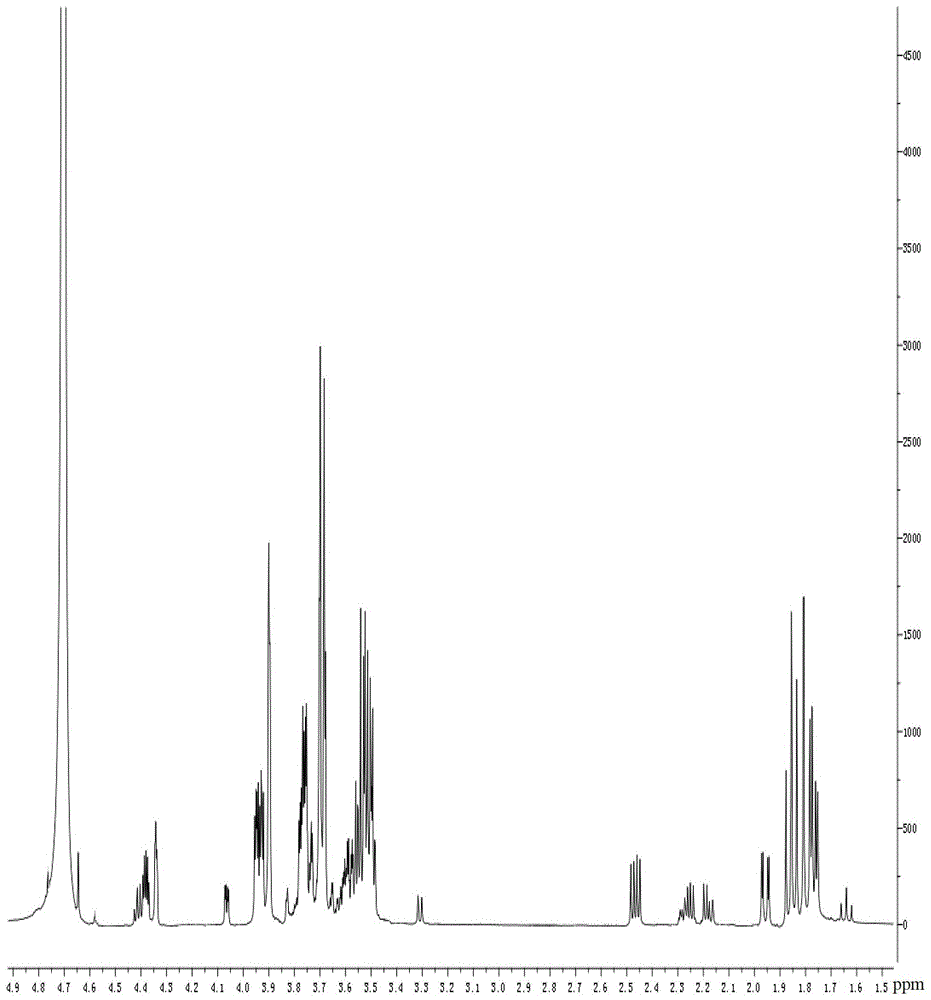
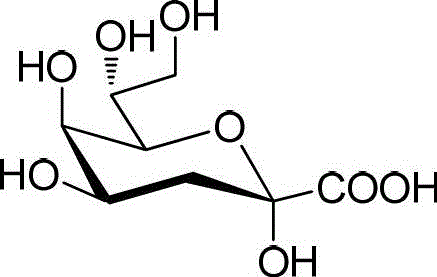
Synthetic method of 3-deoxy-d-mannose-2-octulose acid ammonium salt
A technology for ammonium octyulonic acid salt and synthesis method, which can be applied in the directions of non-glycosyl sugar compounds, chemical instruments and methods, compounds of elements of Group 5/15 of the periodic table, etc., and can solve the problem that the total yield of the route is not high. , environmental pollution, high toxicity and other problems, to achieve the effect of simple operation, easy availability of raw materials and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Taking 10g of raw material D-mannose as an example, the method for synthesizing 3-deoxy-D-mannose-2-octulose acid ammonium salt consists of the following steps:
[0050] (1) Dissolve 10g of D-mannose in 42.7mL of N,N-dimethylformamide, add 27.3mL of 2,2-dimethoxypropane, stir well, add 1.43g of p-toluenesulfonic acid to the solution, Under the catalysis of p-toluenesulfonic acid, react at 25°C for 12 hours, the molar ratio of D-mannose to 2,2-dimethoxypropane, p-toluenesulfonic acid, N,N-dimethylformamide is 1: 4:0.15:10, stop stirring, and further purify, the specific process is: add 3g sodium bicarbonate to the reaction solution, quench the reaction, filter, the filtrate is depressurized by the oil pump to remove N,N-dimethylformamide, add 500mL Diluted with ethyl acetate, washed twice with water, washed once with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated and recrystallized with ethyl acetate to obtain 12.86 g...
Embodiment 2
[0066] Taking 10g of raw material D-mannose as an example, the method for synthesizing 3-deoxy-D-mannose-2-octulose acid ammonium salt consists of the following steps:
[0067] (1) Dissolve 10g of D-mannose in 65mL of N,N-dimethylformamide, add 23.9mL of 2,2-dimethoxypropane, stir well, add 0.95g of p-toluenesulfonic acid to the solution, and Under the catalysis of p-toluenesulfonic acid, react at 25°C for 12 hours, the molar ratio of D-mannose to 2,2-dimethoxypropane, p-toluenesulfonic acid, N,N-dimethylformamide is 1:3.5 :0.1:15, stop stirring, and further purify, the specific process is: add 3g sodium bicarbonate to the reaction solution, quench the reaction, filter, the filtrate is decompressed through the oil pump to remove N,N-dimethylformamide, add 500mL acetic acid Diluted with ethyl ester, washed twice with water, washed once with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated and recrystallized with ethyl acetate ...
Embodiment 3
[0077] Taking 10g of raw material D-mannose as an example, the method for synthesizing 3-deoxy-D-mannose-2-octulose acid ammonium salt consists of the following steps:
[0078] (1) Dissolve 10g of D-mannose in 30mL of N,N-dimethylformamide, add 68mL of 2,2-dimethoxypropane, stir well, add 1.9g of p-toluenesulfonic acid to the solution, Under the catalysis of toluenesulfonic acid, react at 25°C for 12 hours, and the molar ratio of D-mannose to 2,2-dimethoxypropane, p-toluenesulfonic acid, and N,N-dimethylformamide is 1:10: 0.2:7, stop stirring, further purification, the specific process is: add 3g sodium bicarbonate to the reaction solution, quench the reaction, filter, the filtrate is decompressed through the oil pump to remove N,N-dimethylformamide, add 500mL ethyl acetate Ester was diluted, washed twice with water, washed once with saturated brine, dried over anhydrous magnesium sulfate, filtered, and the filtrate was concentrated and recrystallized with ethyl acetate to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com