Container-holding mesylate pazufloxacin aqueous solution and preparation method thereof
A technology of pazufloxacin mesylate and armored methanesulfonic acid, which is applied in the field of aqueous solutions of parenteral drugs, can solve the problems of unqualified foreign objects, achieve good compatibility, and improve stability and safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
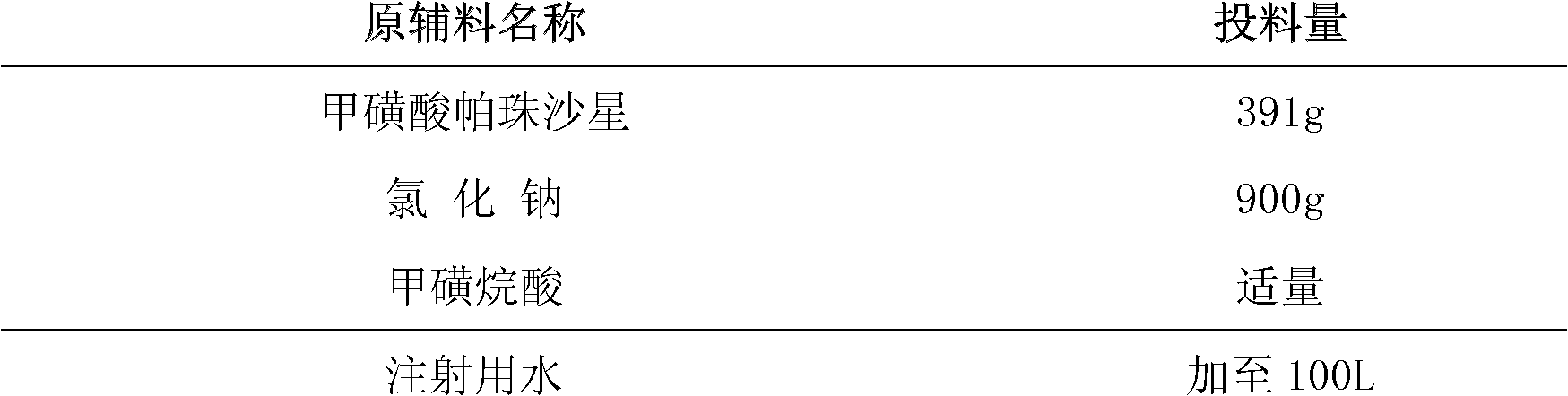
[0015] prescription:
[0016]
[0017] Preparation Process:
[0018] Measure the prescribed amount of water for injection, add the prescribed amount of pazufloxacin mesylate, stir to dissolve, then add the prescribed amount of sodium chloride, after all dissolve, adjust the pH value to 3-4 with methanesulfonic acid, add 0.2 % (g / ml) activated carbon, decarbonized after stirring for 30 minutes, after aseptic filtration, potted in a 100mL glass infusion bottle, sealed with a laminated rubber stopper, and rolled the cap. Autoclave at 117°C for 30 minutes.
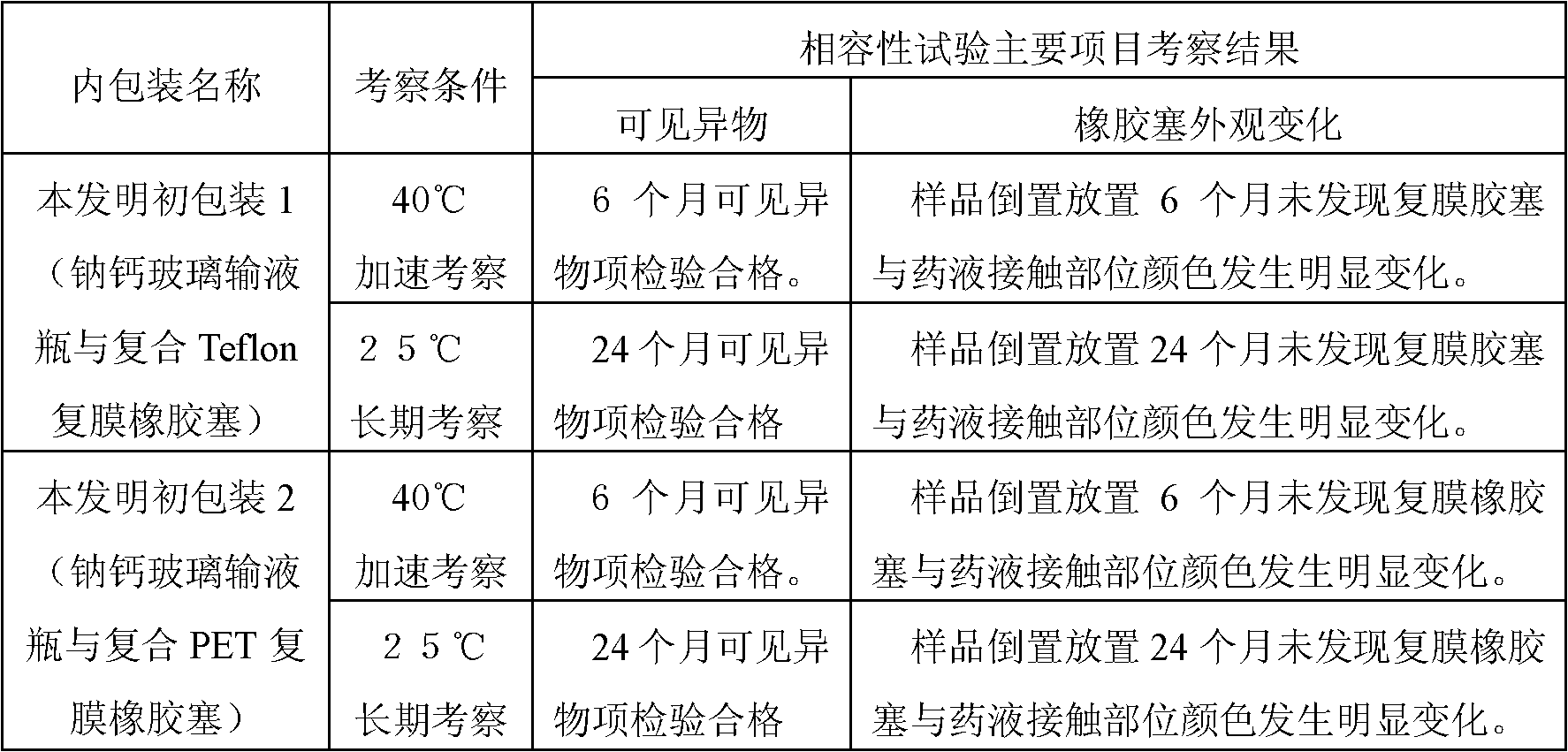
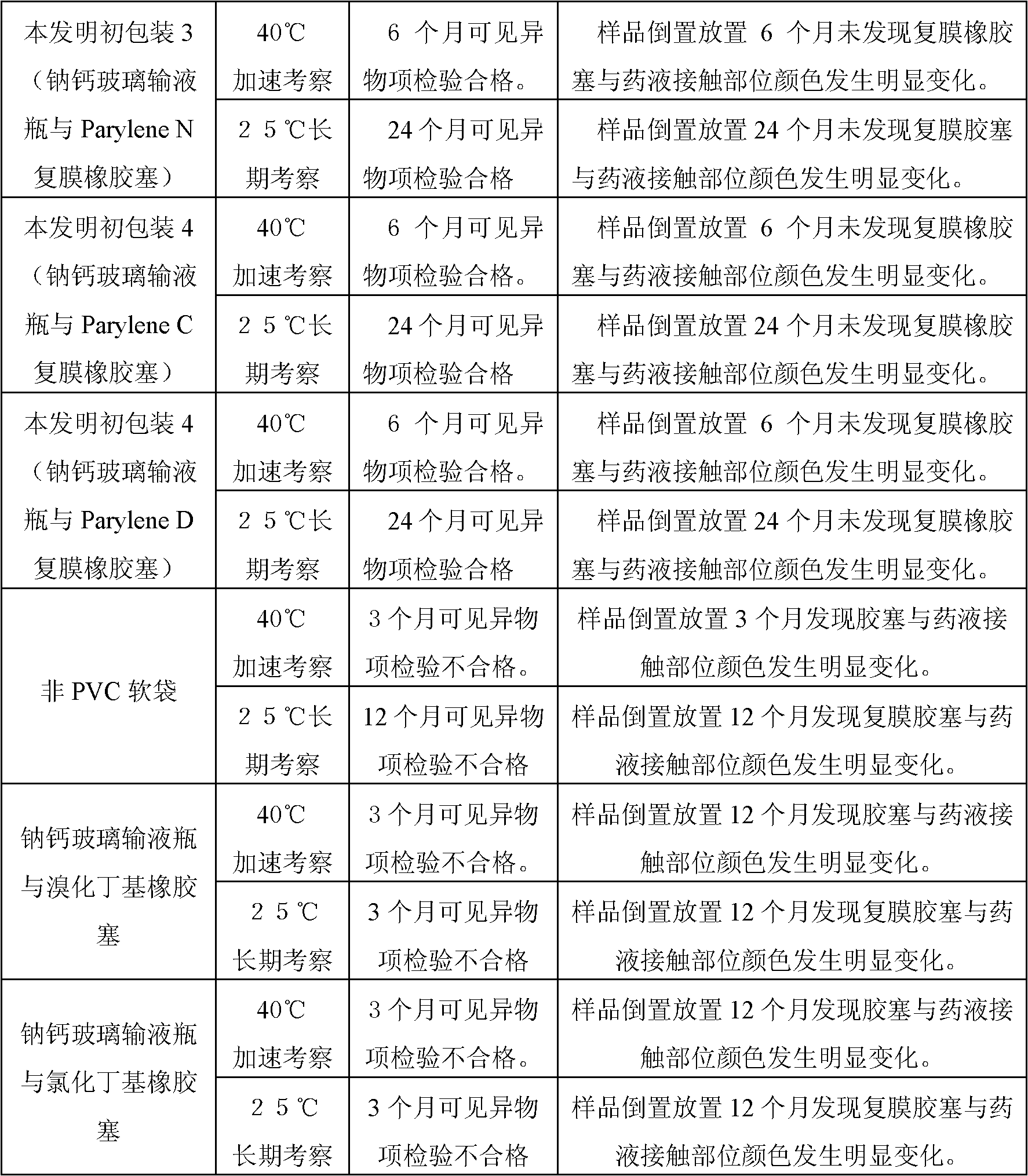
[0019] In order to illustrate the superiority of the present invention, the above-mentioned same medicinal solution was prepared and sealed in the following containers respectively for compatibility experiments, and the visible foreign matter and the appearance change of the rubber stopper were used as the main detection items for preliminary investigation. The test results are shown in Table 1 :
[0020] Table 1. Prelim...
Embodiment 2
[0051] prescription:
[0052]
[0053] Preparation Process:
[0054] Measure the prescribed amount of water for injection, add the prescribed amount of pazufloxacin mesylate, stir to dissolve, then add the prescribed amount of sodium chloride, after all dissolve, adjust the pH value to 3-4 with methanesulfonic acid, add 0.2 % (g / ml) activated carbon, decarbonized after stirring for 30 minutes, after aseptic filtration, potted in a 100mL glass infusion bottle, sealed with a laminated rubber stopper, and rolled the cap. Autoclave at 117°C for 30 minutes.
[0055] In order to illustrate the superiority of the original packaging, the above-mentioned same medicinal solution was prepared and sealed in the following containers respectively, and the compatibility test was carried out, and the visible foreign matter and the appearance change of the rubber stopper were used as the main detection items for preliminary investigation. The test results Table 8 below.
[0056] Table 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com