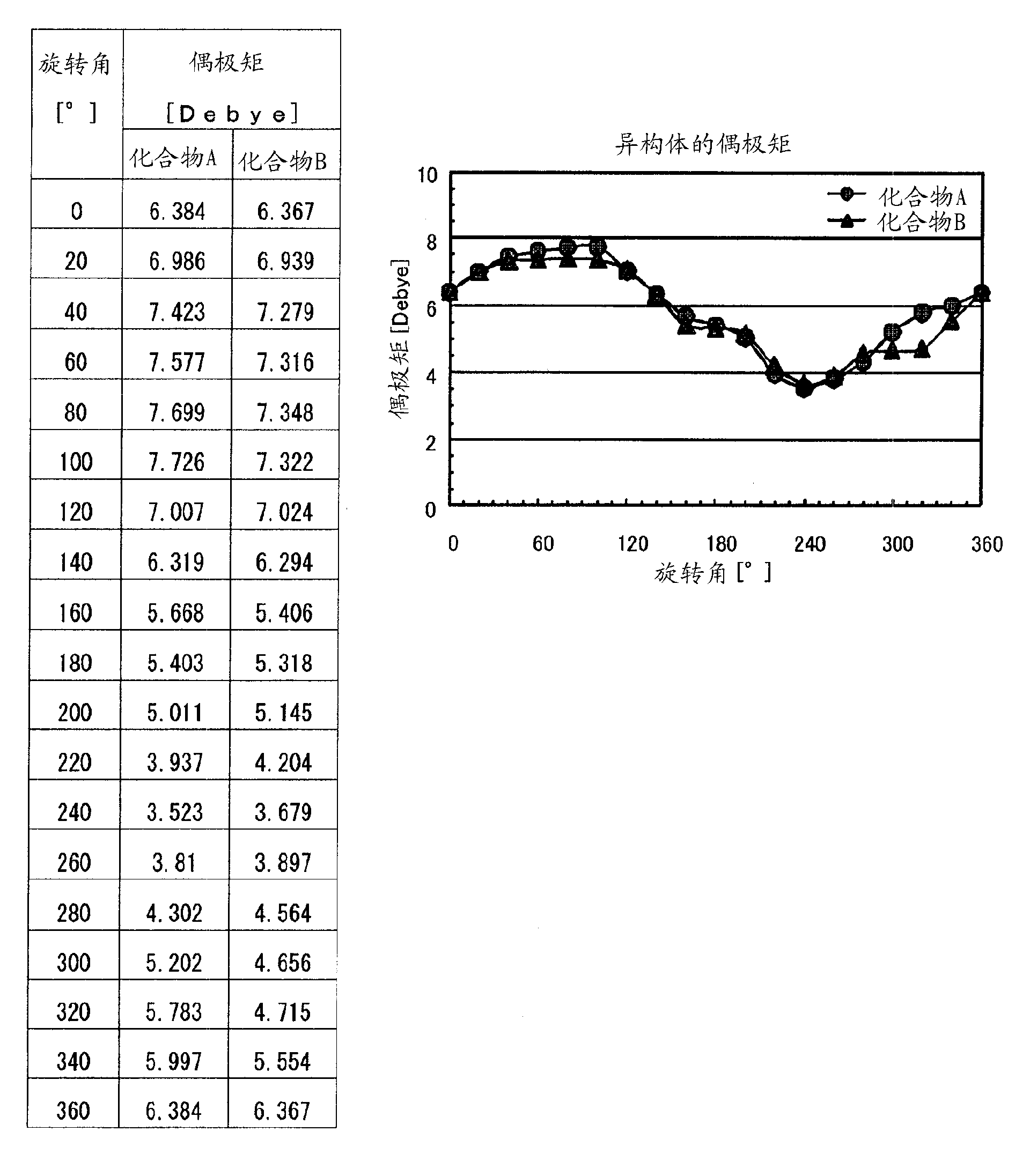
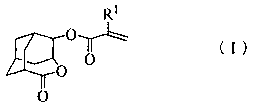
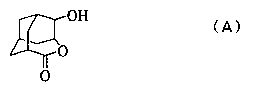Homoadamantane derivatives, process for preparing same, and photoresist compositions
A photoresist and compound technology, applied in the field of new homoadamantane derivatives, can solve problems such as unsatisfactory results, and achieve the effects of excellent exposure sensitivity, reduced roughness, and reduced defects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0078] Examples and comparative examples are shown below, and the present invention will be described more specifically, but the present invention is not limited to them at all.
[0079] In addition, the measuring method of a physical property is as follows.
[0080] (1) Nuclear magnetic resonance spectroscopy (NMR): Measurement was performed with JNM-ECA500 (manufactured by JEOL Ltd.) using deuterated chloroform as a solvent.
[0081] (2) Gas chromatography-mass spectrometry (GC-MS): Measurement was performed using EI (GCMS-QP2010 manufactured by Shimadzu Corporation).
[0082] (3) Weight average molecular weight (Mw), degree of dispersion (Mw / Mn): Measured in terms of polystyrene using an HLC-8220 GPC system (manufactured by Tosoh Corporation, column = TSGgel G-4000HXL + G-2000HXL).
preparation example 1
[0084] (Synthesis of 4-methylsulfonyloxy-2-adamantanone)
[0085] 599.62 g (3.6 mol) of 4-hydroxy-2-adamantanone and 655 mL (4.7 mol) of triethylamine were dissolved in 2.5 L of tetrahydrofuran (THF). Start to slowly add 310 mL (4.0 mol) of methanesulfonyl chloride dropwise thereto. The heat was removed appropriately, the dropwise addition was completed in about 1.5 hours, and the reaction was further performed for 2 hours. 1L of water was added to the reaction solution, and treated by the usual method, 785.74 g (3.2 mol, yield: 89.2%, GC purity: 99.9%) (wherein, Ms represents methylsulfonyl).
[0086] GC-MS: 244 (M + , 11.3%), 165 (15.3%), 148 (27.4%), 120 (43.7%), 91 (29.2%), 79 (100%).
[0087]
preparation example 2
[0089] (Synthesis of endobicyclo[3,3,1]-6-nonene-3-carboxylic acid 1)
[0090] A mixture of 250.52 g (1.0 mol) of 4-methylsulfonyloxy-2-adamantanone synthesized in Preparation Example 1, 460 mL of ethanol, 500 mL (9.5 mol) of 50% aqueous sodium hydroxide solution, and 1.2 L of water was prepared at reflux temperature After reacting for 2 hours, it was cooled to room temperature. Organic impurities contained in the reaction solution were extracted and removed, and then acidified with concentrated hydrochloric acid to precipitate a white solid. The resulting white solid was filtered, and the obtained white filter residue was dissolved with 1.5 L of THF. After the oil and water are separated, they are treated by the usual method to obtain 501.52 g (3.0 mol, yield: 76.4%, GC Purity: 99.2%).
[0091] GC-MS: 166 (M + , 4.7%), 148 (25.4%), 120 (15.5%), 91 (18.9%), 79 (100%).
[0092]
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
| weight-average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com