Use of bacterial beta-lactamase for in vitro diagnostics and in vivo imaging, diagnostics and therapeutics
A lactamase, bacterial technology with applications in pathogenic microbiology and imaging, pharmaceutical fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Bla detection in Mycobacterium tuberculosis in culture
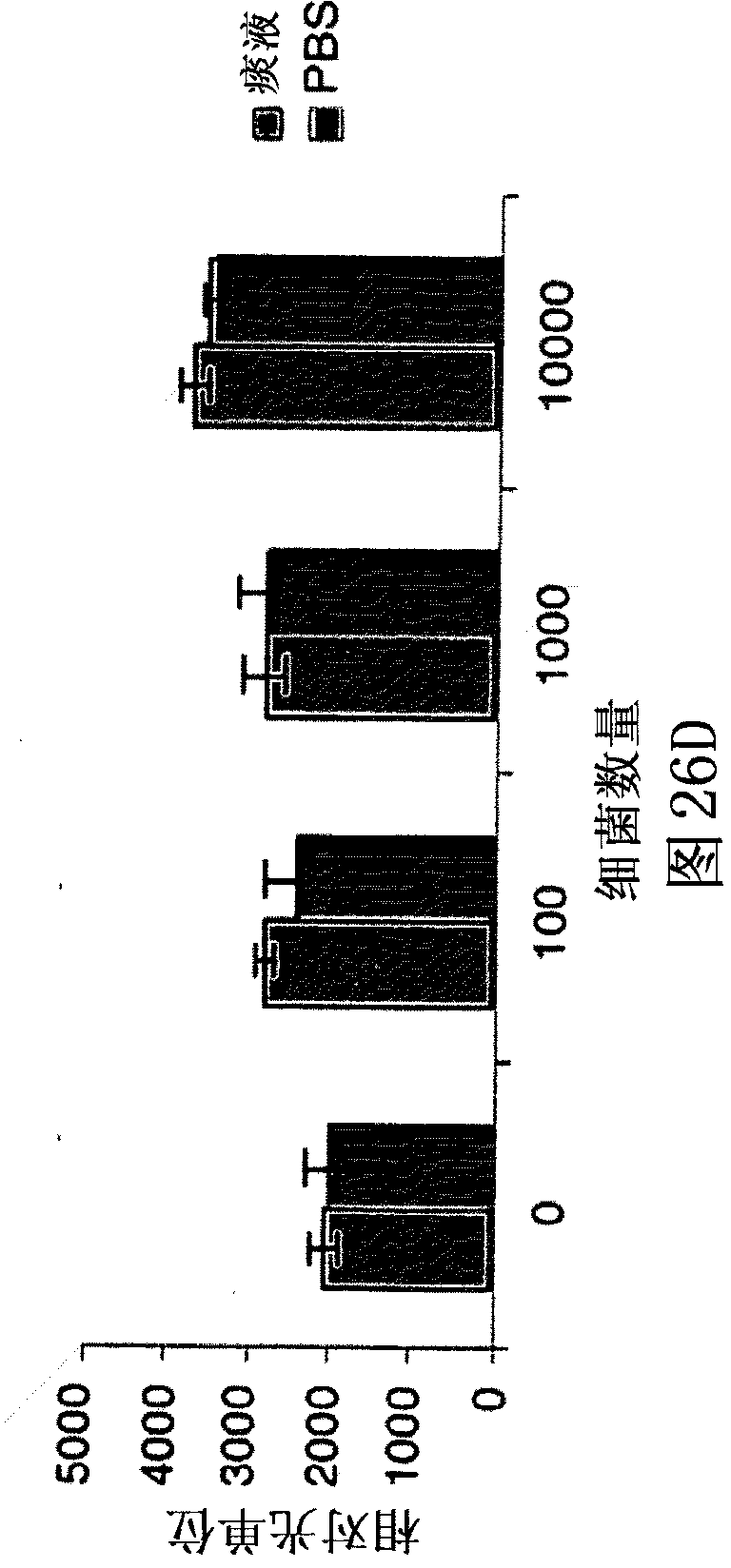
[0105] Using whole cells grown to early log phase and whole cell lysates (lysates), for the detection of Bla in Mycobacterium tuberculosis, compare potential fluorescent gene substrate compounds to known compounds, including nitrocefaxime (Calbiochem), CENTA Bla substrate (Calbiochem), Fluorocillin Green (Molecular Probes), CCF2-AM (Invitrogen) and CCF4-AM (Invitrogen). Dilutions were analyzed for all of these samples to determine the lowest number of bacteria or amount of lysate that produced a significant signal. Potency assays were performed before and after assays with whole cells and prior to lysis for lysates to determine the number of CFUs actually used. Sensitivity and reproducibility were assessed spectrophotometrically in quadruplicate using 96-well plates incubated in bacterial culture medium at 37°C for 15-120 minutes. Initially, compounds were used at the concentrations suggested by the manufactur...
Embodiment 2
[0137] Intra-vital microscopy imaging using cell transplantation models
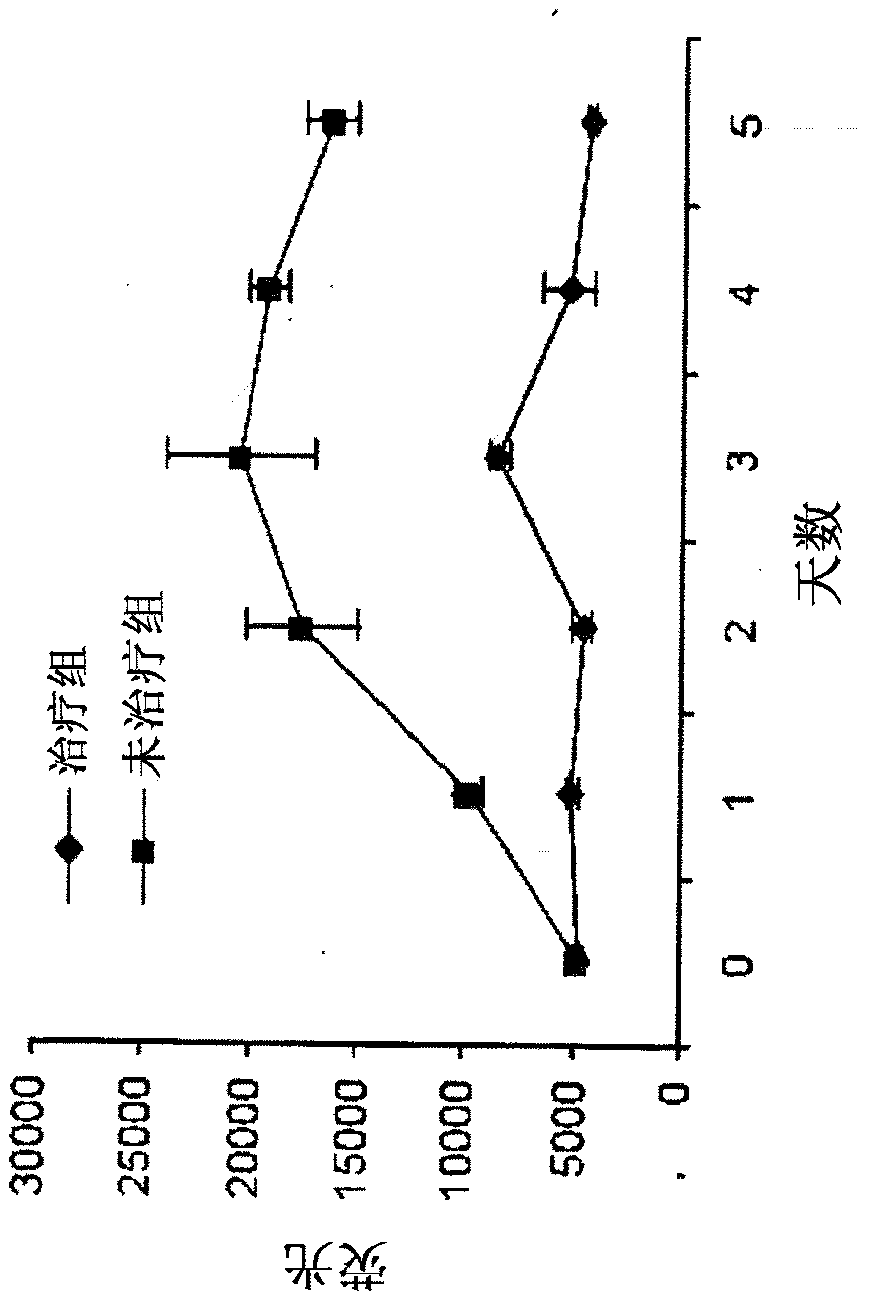
[0138] Generic donor Tr, CD8+ T cells, monocytes, macrophages, and dendritic cells were transplanted into syngeneic mice infected with BCG, and bioluminescence imaging (BLI) in vivo and image-guided biopsy The temporal distribution of these cells was imaged by endoscopy (IVM). A transgenic mouse line in which luciferase is produced through the β-actin promoter provides a source of tissues and cells that will glow in non-transgenic animals (11-12). This mouse line (L2G85), exhibits bright bioluminescence from firefly luciferase (Fluc), and weak GFP fluorescence, so it is in contrast to a single cell that exhibits strong GFP expression and fluorescence in lymphocytes. Germline mating. Thus, after the spatial distribution of the universal donor stem cells and other cells, their exhibited BLI, redistribution or will be cleared in the recipient can be performed and the detected cells can be subsequently o...
Embodiment 3
[0148] Crystallization of enzymes from Mycobacterium tuberculosis BlaC and BlaC mutants
[0149] Very good BlaC crystals were obtained after several months of crystallization. Use 0.1M Tris-HCl, pH8.0, 20.MNH 4 h 2 PO 4 crystallization conditions to prepare co-crystals with penicillin. These crystals allow the visualization of active sites and intermediates of the intact protein, but the initial bound substrates are not visible due to the turnover of the crystal itself. To overcome this obstacle, the Mtb BlaC mutant enzyme was constructed with a mutation in the Glu residue (E166A) involved in hydrolysis, which allowed visualization of specific interactions required for trapping and catalysis of the acy-intermediate on the enzyme. This mutant has been crystallized using a rapid (i.e., about two weeks) crystallization process that yields a high-quality Mtb BlaC mutant ready to be taken up by a substrate ( Figure 1A ). This demonstrates that substrate incorporation into M...
PUM
| Property | Measurement | Unit |
|---|---|---|
| catalytic efficiency | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com