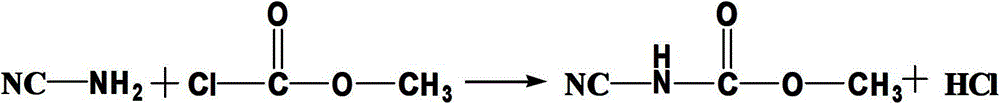
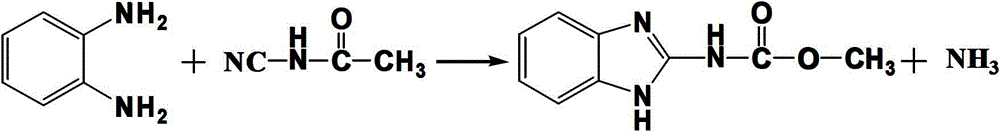
The synthetic method of n-(2-benzimidazolyl)-methyl carbamate
A technology based on methyl carbamate and benzimidazole, applied in the field of organic synthesis, can solve the problems of serious environmental pollution, environmental pollution, complex process, etc., and achieve high yield, simple operation, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A kind of synthetic method of N-(2-benzimidazolyl)-methyl carbamate, its specific steps are:
[0023] (1) Add 11.5g of methyl chloroformate and 16.4g of 50% sodium phosphate solution to 21.0g of 30% cyanamide aqueous solution at the same time. During the dropping process, control the reaction pH value to 7.0, and control the reaction temperature at 5°C. After finishing, heat preservation reaction 2 hours;
[0024] (2) Raise the temperature of the above reaction solution to 95°C, and put 10.8g of o-phenylenediamine into it, keep it warm for 3 hours, heat filter after the reaction, and wash with 80°C water to obtain 17.3g of the product. The obtained product was detected by high performance liquid chromatography (methanol:water=60:40, wavelength 230nm chromatographic column 20RBAX SB-C184.6*150mm5μm, flow rate 1.0ml / min column temperature 30°C). Yield 90.6%, content 98.0%.
Embodiment 2
[0026] A kind of synthetic method of N-(2-benzimidazolyl)-methyl carbamate, its specific steps are:
[0027] (1) Add 14.2g of methyl chloroformate and 11.2g of 50% potassium carbonate aqueous solution to 12.8g of 40% cyanamide aqueous solution at the same time. During the dropping process, control the reaction pH value to 7.2, and control the reaction temperature at 10°C. After finishing, heat preservation reaction 2.3 hours;
[0028] (2) Raise the temperature of the above reaction solution to 93°C, and put 10.8g of o-phenylenediamine into it, keep it warm for 3.5 hours, heat filter after the reaction, and wash with 84°C water to obtain 17.1g of the product. The obtained product was detected by high performance liquid chromatography (methanol:water=60:40, wavelength 230nm chromatographic column 20RBAX SB-C184.6*150mm5μm, flow rate 1.0ml / min column temperature 30°C). Yield 89.5%, content 98.1%.
Embodiment 3
[0030] A kind of synthetic method of N-(2-benzimidazolyl)-methyl carbamate, its specific steps are:
[0031] (1) Add 14.2g of methyl chloroformate and 11.4g of 50% sodium phosphate solution to 14.0g of 30% cyanamide aqueous solution at the same time. During the dropping process, control the reaction pH value to 7.4, and control the reaction temperature at 8°C. After finishing, heat preservation reaction 2.4 hours;
[0032] (2) Raise the temperature of the above reaction solution to 91°C, add 10.8g of o-phenylenediamine into it, keep it warm for 3.2 hours, heat filter after the reaction, and wash with 82°C water to obtain 17.2g of the product. The obtained product was detected by high performance liquid chromatography (methanol:water=60:40, wavelength 230nm chromatographic column 20RBAX SB-C184.6*150mm5μm, flow rate 1.0ml / min column temperature 30°C). Yield 90.3%, content 98.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com