Fusion protein as well as coding gene and application thereof
A fusion protein and gene technology, which is applied in application, genetic engineering, plant genetic improvement, etc., can solve the problem of increased reaction cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
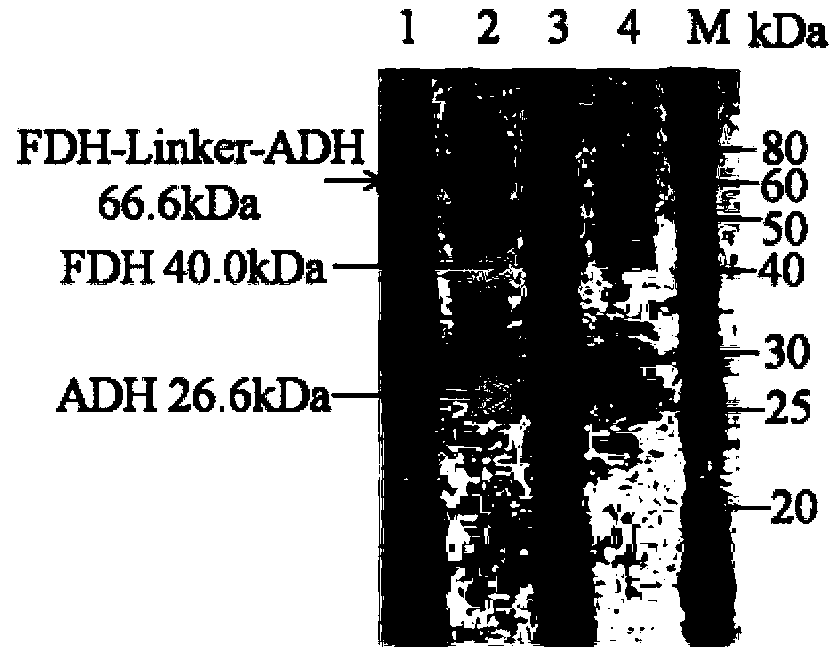
[0030] Embodiment 1, the acquisition of fusion protein and its coding gene
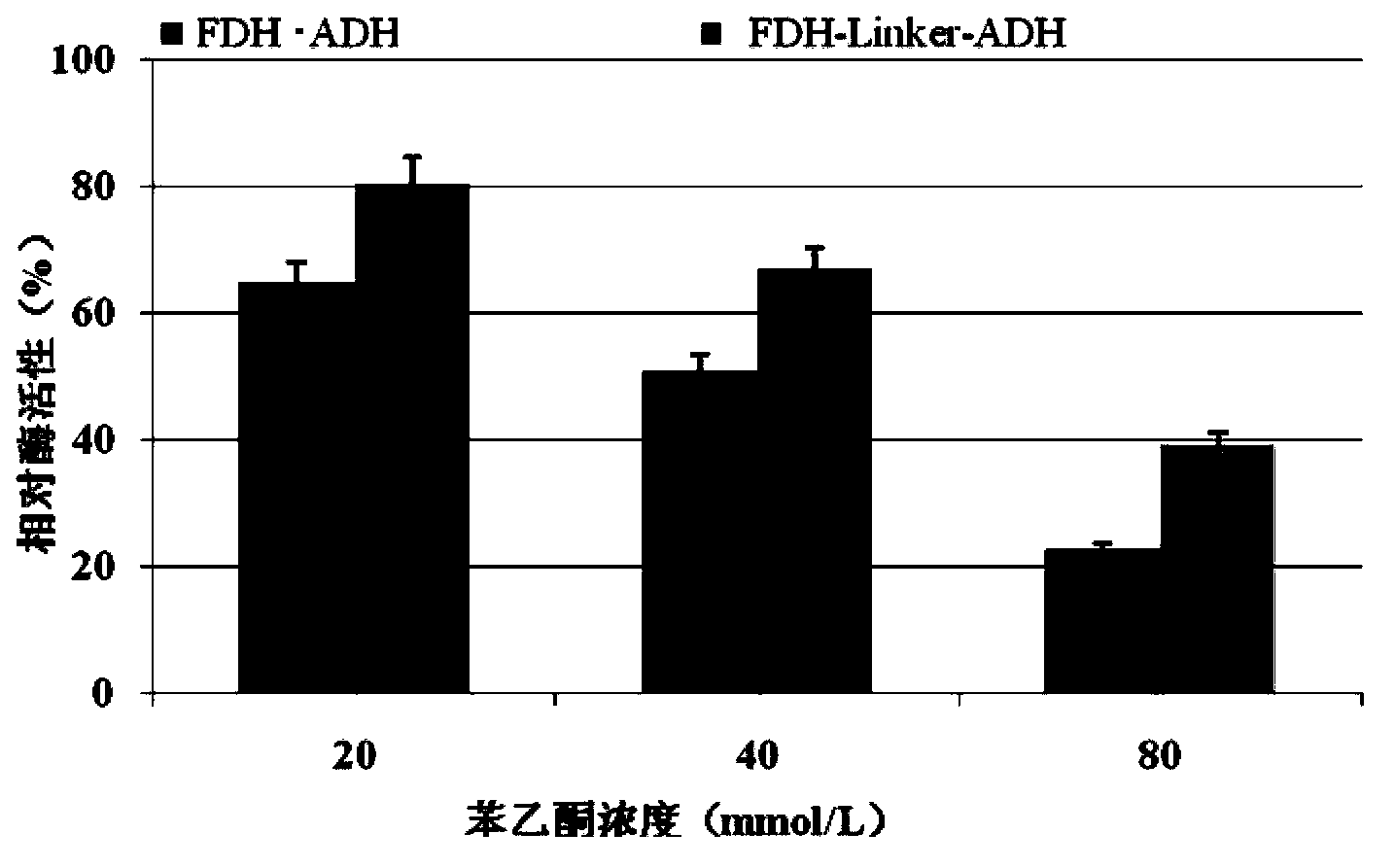
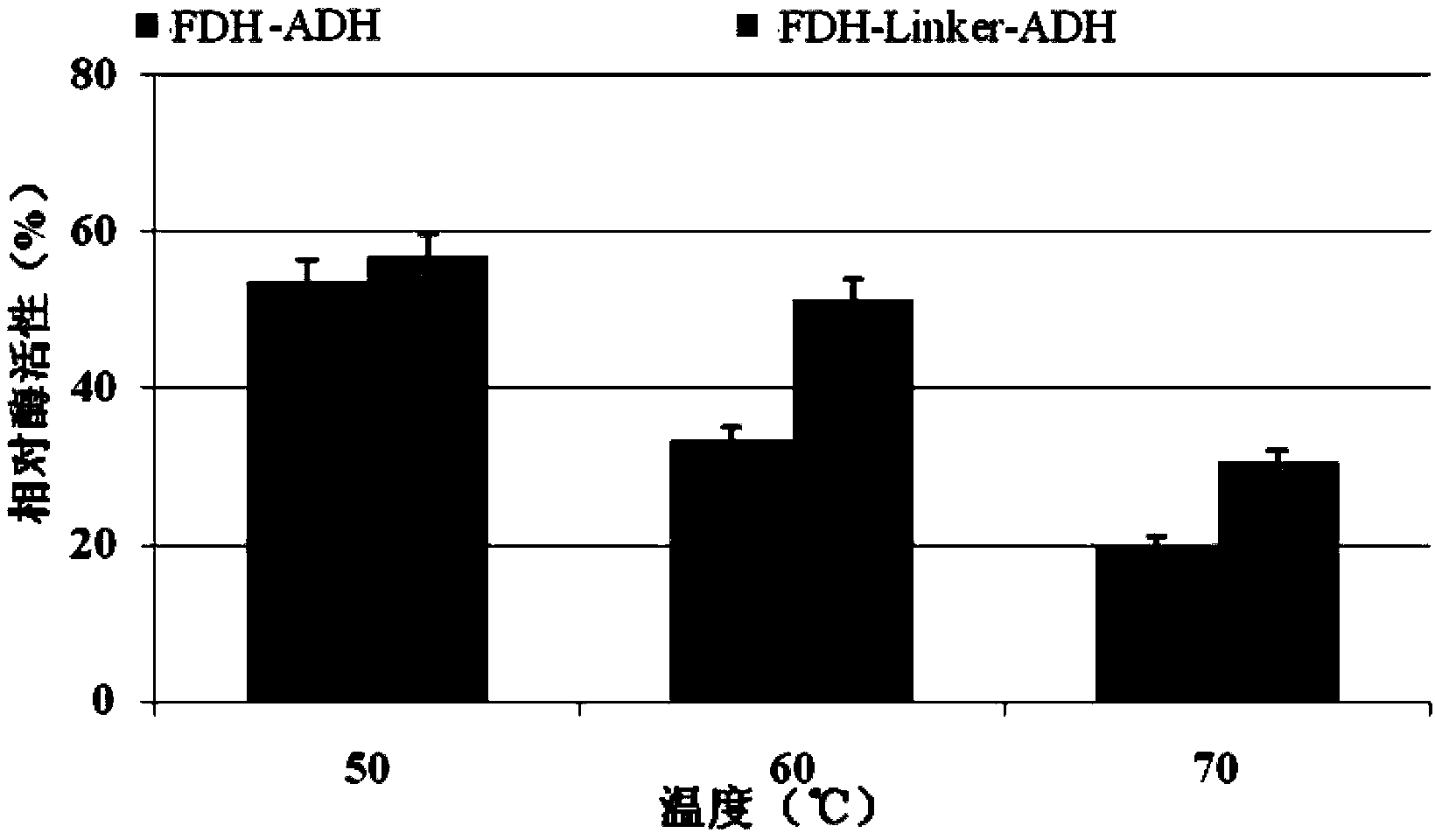
[0031] In this study, alcohol dehydrogenase (ADH) from Lactobacillus brevis with high catalytic activity and strong enantioselectivity and formate dehydrogenase (FDH) from Candida boidinii) were selected according to According to the codon preference principle of Escherichia coli, the codons of the two enzyme genes were optimized separately. A rigid structural linker (EAAAK) will be added between alcohol dehydrogenase and formate dehydrogenase 2 , to obtain the fusion protein FDH-linker-ADH, the amino acid sequence of the fusion protein is sequence 2 in the sequence list. The coding gene of the fusion protein is sequence 1 in the sequence list.
[0032] Wherein the sequence 2 in the sequence listing is alcohol dehydrogenase from the 375th to 625th position of the N' end; the 365th to 374th position from the N' end is a linker; the 1st to 364th position from the N' end is a formate dehydrogenase.
...
Embodiment 2
[0034] Embodiment 2, the application of fusion protein and its coding gene
[0035] 1. Construction of dual-enzyme co-expression vector
[0036] 1. Construction of recombinant vector pETDuet-FDH-ADH
[0037] The FDH-containing vector pGH-FDH (containing the FDH sequence, the entire sequence is sequence 3) was digested with NdeI and Xhol; 3) Skeleton connection to obtain the intermediate vector pETDuet-FDH;
[0038] The ADH-containing vector pGH-ADH (containing the ADH sequence, the full sequence is sequence 4) was digested with BamHI and NotI; the obtained 768bp digested product was connected with the 6484bp intermediate vector pETDuet-FDH backbone that had undergone the same digestion to obtain a recombinant vector pETDuet-FDH-ADH (correctly identified by enzyme digestion).
[0039] 2. Construction of recombinant vector pETDuet-FDH-linker-ADH
[0040] 以pGH-FDH为模板,以FDHF:GACCATGGGCAAAATCGTTCTGGTTCTG,FLAR:TTTGGCCGCTGCTTCTTTGGCCGCTGCTTCTTTTT TGTCGTGTT为引物,得到1124bp的PCR产物;以pGH-A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com