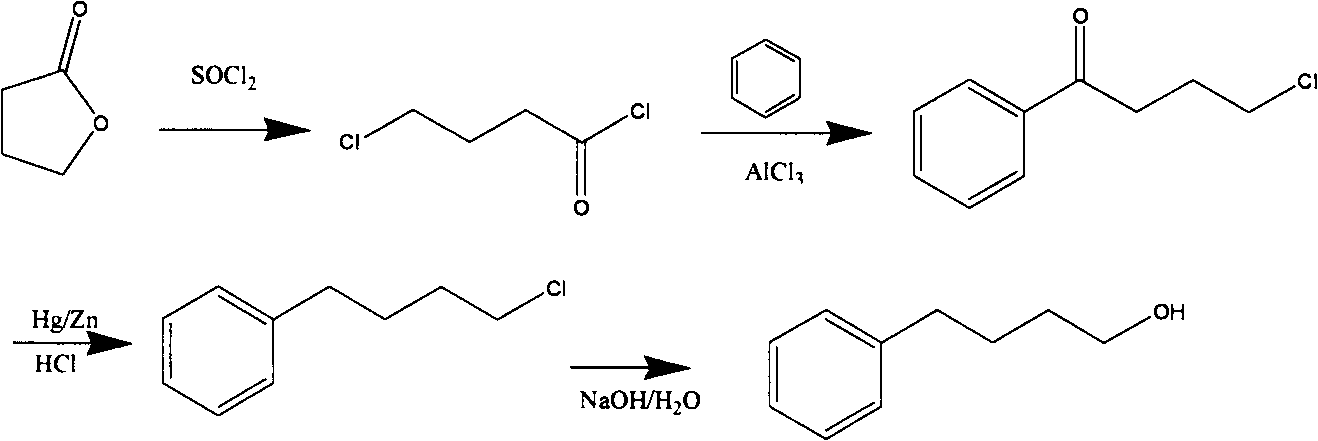
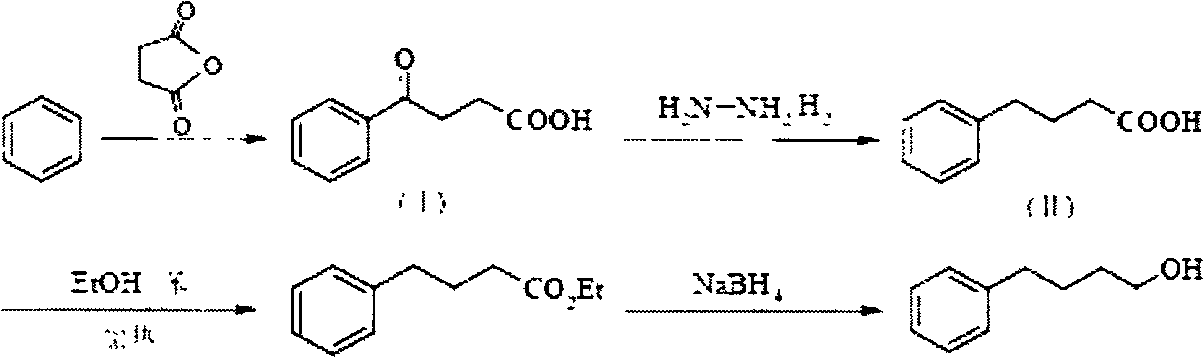
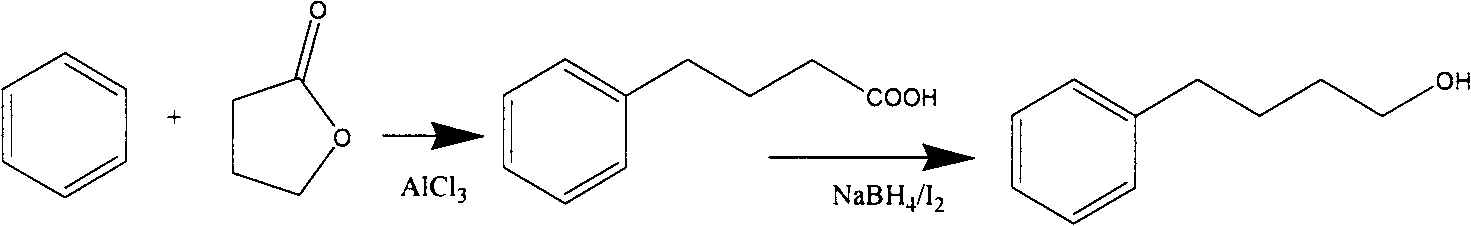
Novel synthesis process for 4-phenyl-1-butanol
A technology of phenyl and synthetic formula, which is applied in the field of medicine, can solve the problems of strong corrosion of equipment, low cost of raw materials, difficulty in obtaining high-purity 4-chlorobutanol, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 1 g of anhydrous zinc chloride and 78.5 g of acetyl chloride to a 250 ml three-necked flask, add 72 g of tetrahydrofuran dropwise under stirring, cool in an ice bath to keep the temperature below 30 degrees, keep warm for 1 hour after dropping, and distill under reduced pressure with a water pump 145 g of ethyl 4-chlorobutanol was obtained with a yield of 96% and a purity of 99.5%.
[0032] Add 30 grams of ethyl 4-chlorobutanol and 78 grams of benzene into a 250-milliliter three-necked flask respectively, cool to 0 degrees under stirring, add 30 grams of anhydrous aluminum trichloride in batches, and control the temperature below 10 degrees. After the addition, the temperature was kept at 10°C for 3 hours, and the reaction of the raw materials was detected by gas phase detection. The reaction solution was slowly poured into crushed ice for hydrolysis, stirred for 30 minutes, and the organic phase was separated. The organic phase was washed with water and saturated ...
Embodiment 2
[0034] Add 1 gram of anhydrous zinc chloride and 78.5 grams of acetyl chloride to a 1000 ml three-necked flask, add 72 grams of tetrahydrofuran dropwise under stirring, cool in an ice bath to keep the temperature below 30 degrees, and keep warm for 1 hour after the drop to complete the reaction. Gas phase detection The content of 4-chlorobutanol acetate is 97%. 390 g of benzene was added to the reaction solution, and the temperature was lowered to 0°C. Add 160 grams of aluminum trichloride in batches, keeping the temperature below 10 degrees. After insulated for 3 hours, the raw materials were detected by gas chromatography and disappeared. The reaction solution was slowly poured into ice water for hydrolysis, stirred for 30 minutes, the organic phase was separated, washed with water and saturated brine, and dried over anhydrous magnesium sulfate. After the benzene was removed by distillation, the oil pump decompressed distillation to obtain 144 grams of colorless liquid phe...
Embodiment 3
[0036] Add 1 gram of anhydrous zinc chloride and 141 grams of benzoyl chloride to a 250 ml three-necked bottle, add 72 grams of tetrahydrofuran dropwise at room temperature under stirring, keep it stable below 40 degrees during the dropping process, keep warm for 2 hours after the dropping, and depressurize Distilled to obtain 200 g of 4-chlorobutanol benzoate with a yield of 94% and a purity of 99.35%.
[0037] Add 42.5 grams of 4-chlorobutanol benzoate and 78 grams of benzene into a 250-milliliter three-neck flask respectively, cool to 0 degrees under stirring, add 30 grams of anhydrous aluminum trichloride in batches, and control the temperature below 10 degrees. After the addition, the temperature was kept below 10 degrees for 3 hours, and the reaction of the raw materials was detected by gas phase detection. The reaction solution was slowly poured into crushed ice for hydrolysis, stirred for 30 minutes, and the organic phase was separated. The organic phase was washed wi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com