Ketobutyric acid and preparation method for ketobutyric acid salt
A technology of methyl butyronic acid and oxobutyrate, which is applied in the preparation of carboxylic acid esters, organic compounds, carboxylic acid esters/lactones, etc., can solve unfavorable industrial production and wide application, and low selectivity , volatile and other issues, to achieve the effect of being suitable for large-scale industrial applications, high yield and high purity, high purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
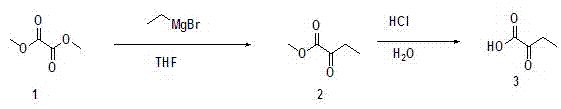
[0025] Example 1 Preparation of 2-butanuonic acid.
[0026] Dissolve 118 g of dimethyl oxalate in 2000 mL of anhydrous tetrahydrofuran, cool to -78°C, add 500 mL of 1 mol / L ethylmagnesium bromide solution, stir, react for 2 hours, and distill off the solvent under reduced pressure, leaving The mixture was then rectified under reduced pressure, and the intermediate product 2-oxobutyric acid methyl ester was collected to obtain 50 g, and the yield of 2-oxobutyric acid methyl ester was 76%. Dissolve 50 g of methyl 2-oxobutyrate collected in 2.5 L of 1 mol / L hydrochloric acid, heat to reflux, and stir for 2 h. After the reaction is monitored by TLC, extract three times with 3 L of ethyl acetate, and collect The organic phase was dried by adding anhydrous magnesium sulfate, filtered, and the filtrate was distilled off under reduced pressure to obtain 43 g of crude product 2-butanuonic acid. The preparation process is as figure 1 As shown, the yield of 2-butyronic acid prepared ...
Embodiment 2
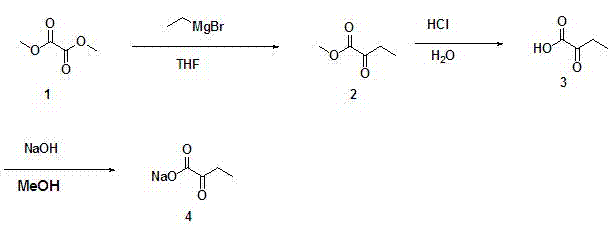
[0030] Example 2 Preparation of sodium 2-butanone.
[0031] Weigh 20 g of the crude product 2-butanuonic acid prepared in Example 1, dissolve it in 50 mL of methanol, cool to 0 °C, add 70 mL of 10% sodium hydroxide methanol solution, and react at this temperature for 10 min. Then the solvent was distilled off under reduced pressure at 25°C, the remaining solid was washed with 500 mL of ethyl acetate, and filtered to obtain a white solid. The solid was dried in a vacuum oven at room temperature for 2 h to obtain 17 g of the final product, and the yield of sodium 2-butyruvate prepared from 2-butanuonic acid was 70%.
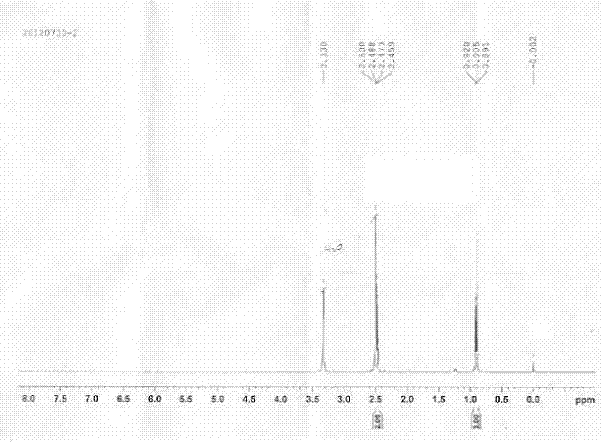
[0032] Therefore, the total yield of the preparation method of sodium 2-butyruvate provided by the invention is 52.1%, and the preparation process is as follows figure 2 shown. The prepared sodium 2-butanone is detected, and the result is as follows: image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com