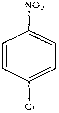
Method for preparing p-nitrochlorobenzene by nitrifying chlorobenzene by using nitrogen dioxide
A technology of p-nitrochlorobenzene and nitrogen dioxide, which is applied in the preparation of nitro compounds and organic chemistry, can solve the problems of large fluctuations in demand for nitrochlorobenzene and serious environmental pollution, and achieve good nitration product selectivity, Reduction of environmental pollution and high safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] After stirring 5.5g of chlorobenzene, 0.55g of HZSM-5 type catalyst, and 0.11g of 3? molecular sieve evenly, pass in 0.15mL of nitrogen dioxide, stir magnetically in an oxygen atmosphere, and react at 15°C for 48h. After the reaction was completed, the reaction was terminated with deionized water to obtain a crude reaction product. Filter the crude product with a glass funnel to remove the catalyst and molecular sieve, and let stand to separate the organic phase. The organic phase was washed successively with sodium bicarbonate solution and distilled water several times until it became neutral, and 6.13 g of nitrochlorobenzene was obtained by distillation under reduced pressure, with a yield of 79.6%. After drying, nitrobenzene was used as an internal standard for high-performance liquid chromatography analysis. The internal standard method was used to calculate the mass of p-nitrochlorobenzene to be 5.52 g, and the mass fraction was 90.0%.
[0024]
Embodiment 2
[0026] Stir 5.5g of chlorobenzene, 1g of CuHZSM-5 catalyst, and 0.25g of 3? molecular sieve evenly, then inject 0.3mL of nitrogen dioxide, stir magnetically in a nitrogen atmosphere, and react at 30°C for 36h. After the reaction was completed, the reaction was terminated with deionized water to obtain a crude reaction product. Filter the crude product with a glass funnel to remove the catalyst and molecular sieve, and let stand to separate the organic phase. The organic phase was washed with 5% sodium bicarbonate solution and distilled water several times until it was neutral, and 6.34 g of nitrochlorobenzene was obtained by distillation under reduced pressure, with a yield of 82.3%. After drying, HPLC analysis was carried out with nitrobenzene as the internal standard. The mass of p-nitrochlorobenzene was calculated to be 5.43 g and the mass fraction was 85.7 % by internal standard method.
[0027]
Embodiment 3
[0029] Stir 5.5g of chlorobenzene, 2g of FeHZSM-5 catalyst, and 0.45g of 3? molecular sieve evenly, then inject 0.5mL of nitrogen dioxide, stir magnetically in an argon atmosphere, and react at 60°C for 24h. After the reaction was completed, the reaction was terminated with deionized water to obtain a crude reaction product. Filter the crude product with a glass funnel to remove the catalyst and molecular sieve, and let stand to separate the organic phase. The organic phase was washed with sodium bicarbonate solution and distilled water several times until it became neutral, and 6.04 g of nitrochlorobenzene was obtained by distillation under reduced pressure, with a yield of 78.4%. After drying, nitrobenzene was used as an internal standard for high-performance liquid chromatography analysis. The mass of p-nitrochlorobenzene was calculated to be 5.10 g and the mass fraction was 84.5 % by internal standard method.
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com