A kind of lithium iron phosphate cathode material and preparation method thereof
A technology for lithium iron phosphate and positive electrode materials, applied in chemical instruments and methods, phosphorus compounds, battery electrodes, etc., can solve the problems of low ion diffusion speed, one-dimensional ion conduction, poor cycle performance and rate performance, etc. No agglomeration, excellent low temperature performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
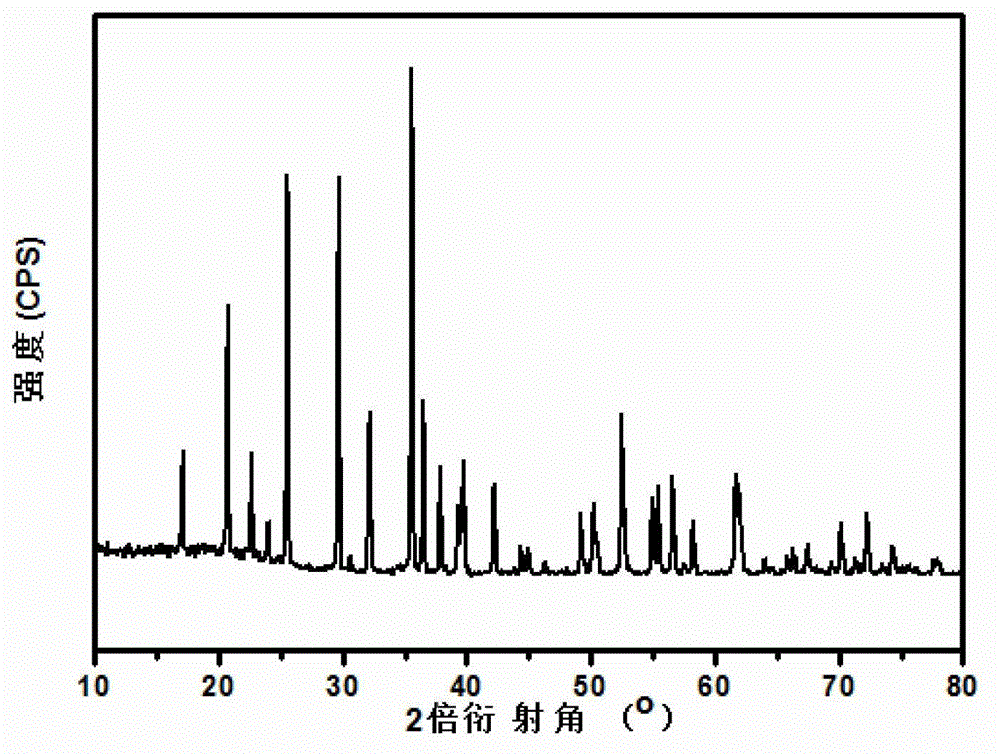
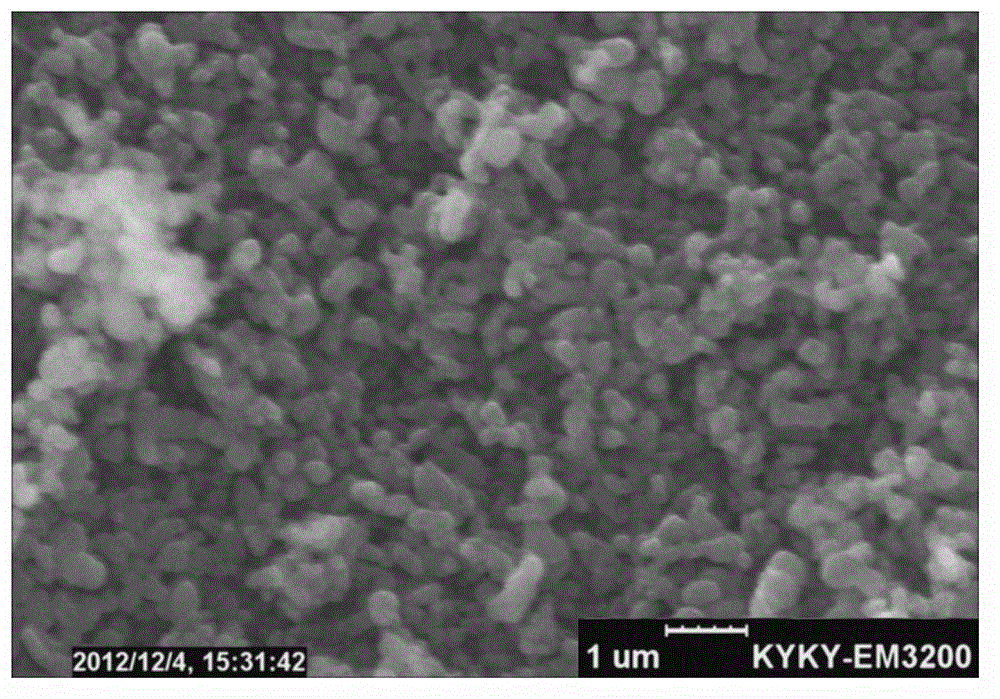
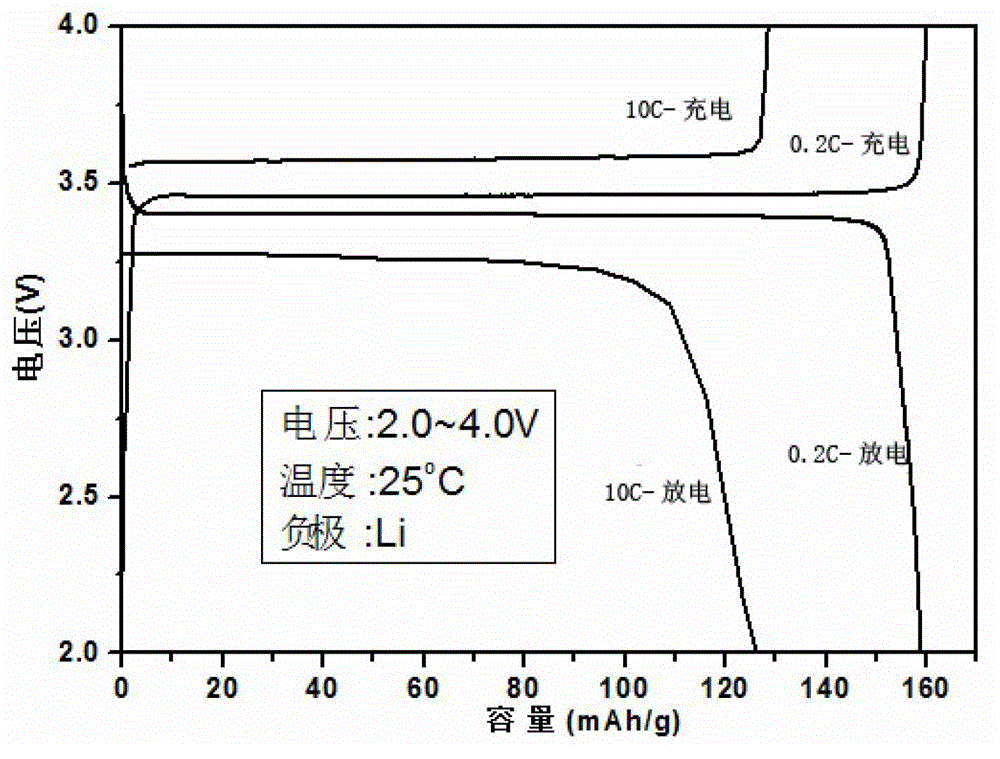
Image
Examples
Embodiment 1
[0036] Weigh 1.68g of lithium carbonate, 8.03g of ferrous oxalate dihydrate and 5.16g of phosphoric acid with a concentration of 85%, wherein the molar ratio Li:Fe:P=1.02:1:1, fully mixed and dried at 100°C for 3 hours A dry material is obtained. A dry material was calcined in a nitrogen atmosphere at 500° C. for 8 hours, and cooled with the furnace to obtain 7.04 g of a calcined material. Add the first-calcined material, 0.11g of lithium carbonate, 0.30g of ferrous oxalate dihydrate and 0.17g of phosphoric acid (Li:Fe:P=2:1:2) with a concentration of 85% into 50ml of acetone, and mix them thoroughly at 110 Drying at °C for 2.5 hours gave a dry material. The two-calcined material was calcined in a nitrogen atmosphere at 600° C. for 8 hours, and cooled with the furnace to obtain 7.22 g of the two-calcined material. The dioxane material and 0.36 g of glucose were added to 50 ml of acetone, mixed thoroughly and then dried at 120° C. for 2 hours to obtain a tri-dry material. The ...
Embodiment 2
[0040] Weigh 42.16g of lithium hydroxide, 77.14g of ferric oxide, 111.14g of ammonium dihydrogen phosphate and 3.29g of tetrabutyl titanate into 700ml of deionized water, wherein the molar ratio Li:Fe:P:Ti=1.04:1 :1:0.01, mixed thoroughly and dried at 90°C for 5 hours to obtain a dry material. A dry material was calcined in an argon atmosphere at 600° C. for 4 hours, and cooled with the furnace to obtain 152.43 g of a calcined material. Add the first-calcined material, 2.10g lithium hydroxide, 2.00g ferric oxide and 5.76g ammonium dihydrogen phosphate (Li:Fe:P=2:1:2) into 700ml deionized water, mix well and set at 150°C Drying was carried out for 2 hours to obtain a dry material. Calcined the two-calcined material in an argon atmosphere at 500° C. for 9 hours, and cooled with the furnace to obtain 157.61 g of the two-calcined material. Add the two-calcined material and 12g of rock sugar into 700ml of deionized water, mix well and dry at 120°C for 2 hours to obtain the three-...
Embodiment 3
[0042] Weigh 53.52g of anhydrous lithium acetate, 178.20g of ferric oxalate pentahydrate, 101.04g of diammonium hydrogen phosphate and 0.78g of aluminum oxide and add them to 1000ml of acetone, wherein the molar ratio Li:Fe:P:Al=1.06:1: 1:0.02, mixed thoroughly and dried at 200°C for 2 hours to obtain a dry material. A dry material was calcined in a nitrogen atmosphere at 550° C. for 6 hours, and cooled with the furnace to obtain 120.70 g of a calcined material. Add the first-calcined material, 1.96g of anhydrous lithium acetate, 3.46g of ferric oxalate pentahydrate and 3.93g of diammonium hydrogen phosphate (Li:Fe:P=2:1:2) into 1000ml of acetone, mix well and set at 160°C Drying was carried out for 2 hours to obtain a dry material. Calcined the two-dried material in a nitrogen atmosphere at 700° C. for 5 hours, and cooled with the furnace to obtain 123.61 g of the two-calcined material. Add the two-burned material and 12g of asphalt into 1000ml of acetone, mix well and dry ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com