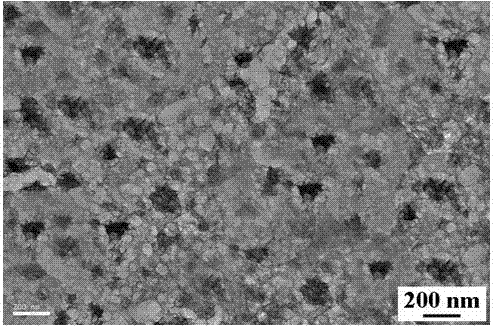
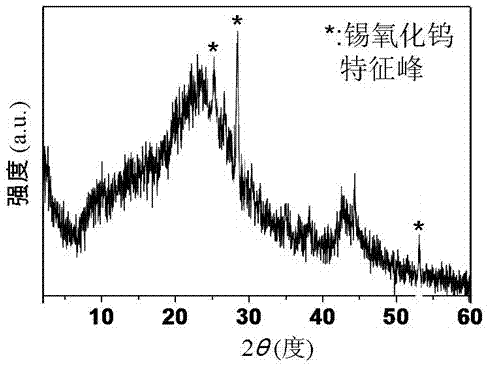
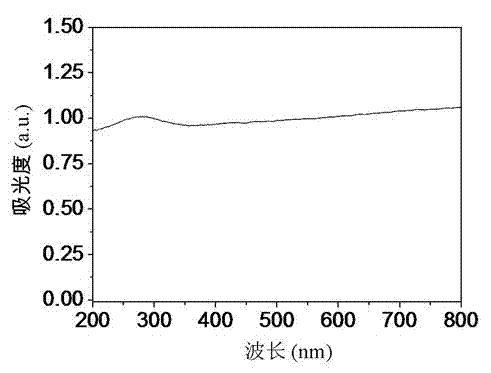
Novel nano-composite visible light catalyst and preparation method thereof
A nanocomposite, visible light technology, applied in the field of photocatalytic materials, can solve the problems of uncontrollable tin tungsten oxide particle size, decreased photocatalytic activity, and decreased specific surface area, so as to improve catalyst activity, reduce recombination rate, and promote separation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Weigh 1 g of graphene oxide prepared by the modified Hummer method, add 5 g of NaOH, grind it evenly, and then place it in a tube furnace and heat it at 760 °C for 1 h under a nitrogen atmosphere, with a programmed temperature increase rate of 5 °C / min, to obtain Porous graphene; the specific surface area of porous graphene is 350 m 2 / g; its conductivity is 60 S m -1 ; The weight loss at 900 ℃ is 4 wt%.
[0026] 22.6 mg of SnCl 2 2H 2 O and 33 mg of Na 2 WO 4 2H 2 The O solid (weighed according to the mass ratio of 1:1) was successively added to 50 mL of deionized water, then 1 mg of anhydrous sodium acetate and 1 mg of ethylene glycol were added, and magnetically stirred for 30 min to obtain the precursor.
[0027] Move the precursor into a reaction tank, put it into a stainless steel reaction kettle, add 1 g of porous graphene, seal it, put it in an oven, and react at 170 °C for 6 h. After the reaction, it was cooled to room temperature to obtain a yellow-bl...
Embodiment 2
[0033] Weigh 1 g of graphene oxide prepared by the modified Hummer method, add 4 g of KOH, grind it evenly, and then place it in a tube furnace and heat it at 760 °C for 1 h under a nitrogen atmosphere, with a programmed temperature increase rate of 5 °C / min, to obtain Porous graphene; the specific surface area of porous graphene is 370 m 2 / g; its conductivity is 50 S m -1 ; The weight loss at 900 ℃ was 4.5 wt%.
[0034] 22.6 mg of SnCl 2 2H 2 O and 33 mg of Na 2 WO 4 2H 2 The O solid (weighed according to the mass ratio of 1:1) was successively added to 75 mL of deionized water, then 1.5 mg of anhydrous sodium acetate and 3 mg of ethylene glycol were added, and magnetically stirred for 30 min to obtain the precursor.
[0035] Move the precursor into a reaction tank, put it into a stainless steel reaction kettle, add 1.5 g of porous graphene, seal it, put it in an oven, and react at 180 °C for 7 h. After the reaction, it was cooled to room temperature to obtain a yel...
Embodiment 3
[0038] Weigh 1 g of graphene oxide prepared by the improved Hummer method, add 3 g of NaOH, grind evenly, and then heat in a tube furnace at 760 °C for 1 h under nitrogen protection, with a programmed temperature rise rate of 5 °C / min, to obtain porous graphene; Porous graphene has a specific surface area of 390 m 2 / g; its conductivity is 40 S m -1 ; The weight loss at 900 ℃ is 5 wt%.
[0039] 22.6 mg of SnCl 2 2H 2 O and 33 mg of Na 2 WO 4 2H 2 The O solid (weighed according to the mass ratio of 1:1) was successively added to 75 mL of deionized water, then 2 mg of anhydrous sodium acetate and 1 mg of ethylene glycol were added, and magnetically stirred for 30 min to obtain the precursor.
[0040] Move the precursor into a reaction tank, put it into a stainless steel reaction kettle, add 2 g of porous graphene, seal it, put it in an oven, and react at 180 °C for 8 h. After the reaction, it was cooled to room temperature to obtain a yellow-black precipitate. After th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com