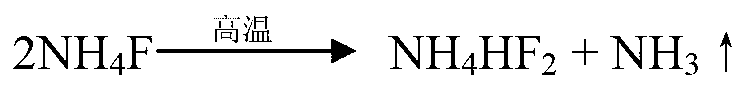Method for preparing potassium fluoride by utilizing potassium fluosilicate
A technology of potassium fluorosilicate and potassium fluoride, which is applied in the field of inorganic chemical production, can solve the problems of few utilization routes of crude potassium fluorosilicate, large environmental impact, environmental pollution, etc., so as to solve the problems of development, utilization and environmental protection, and reduce impurities. , the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for preparing potassium fluoride with potassium fluorosilicate, the method may further comprise the steps:
[0037] (1) The crude potassium fluorosilicate is washed with hot water (60°C) to neutrality, and prepared into a potassium fluorosilicate slurry with a concentration of 30% by mass, and 2000 kg of potassium fluorosilicate slurry is slowly added to the In an ammonia solution with a concentration of 25% by mass, the molar ratio of potassium fluorosilicate to ammonia is 1:7.0, the temperature is 60°C, and the reaction is performed for 1 hour to obtain potassium fluoride solution, ammonium fluoride solution and silicon dioxide solid. mixed slurry;
[0038](2) Let the mixed slurry settle for 2 hours, and then filter to obtain a filter cake of solid silica and a filtrate containing potassium fluoride and ammonium fluoride; The effluent is neutral, and the specific surface area is 177m3 after drying. 2 261 kilograms of finished white carbon black of / g;
[0...
Embodiment 2
[0041] A method for preparing potassium fluoride with potassium fluorosilicate, the method may further comprise the steps:
[0042] (1) The crude potassium fluorosilicate is washed with hot water (70°C) to neutrality, and prepared into a potassium fluorosilicate slurry with a concentration of 20% by mass. Take 2000 kg of potassium fluorosilicate slurry and slowly add it to the In an ammonia solution with a concentration of 10% by mass, the molar ratio of potassium fluorosilicate to ammonia is 1:8.0, and the temperature is 70°C for 0.5 hours to obtain a mixture containing potassium fluoride solution, ammonium fluoride solution and silica solid. slurry;
[0043] (2) Settling the mixed slurry for 3 hours, filtering to obtain a filter cake of solid silica and a filtrate containing a mixed solution of potassium fluoride and ammonium fluoride; the filter cake was washed 3 times with water until it was washed out The liquid is neutral, and the specific surface area is 200m2 after dr...
Embodiment 3
[0046] A method for preparing potassium fluoride with potassium fluorosilicate, the method may further comprise the steps:
[0047] (1) The crude potassium fluorosilicate is washed with hot water (60°C) to neutrality, and prepared into a potassium fluorosilicate slurry with a concentration of 50% by mass. Take 2000 kg of potassium fluorosilicate slurry and slowly add it to the In an ammonia solution with a concentration of 20% by mass, the molar ratio of potassium fluorosilicate to ammonia is 1:9.0, and the temperature is 65°C for 2 hours to obtain a mixture containing potassium fluoride solution, ammonium fluoride solution and silica solid. slurry;
[0048] (2) Settling the mixed slurry for 4 hours, filtering to obtain a filter cake of solid silica and a filtrate containing a mixed solution of potassium fluoride and ammonium fluoride; the filter cake was washed 4 times with water until it was washed out The liquid is neutral, and the specific surface area after drying is 131...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com