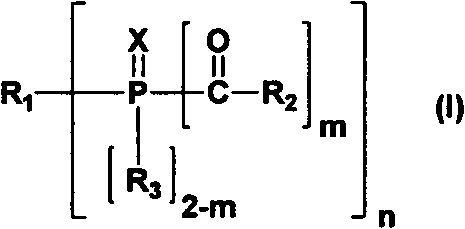
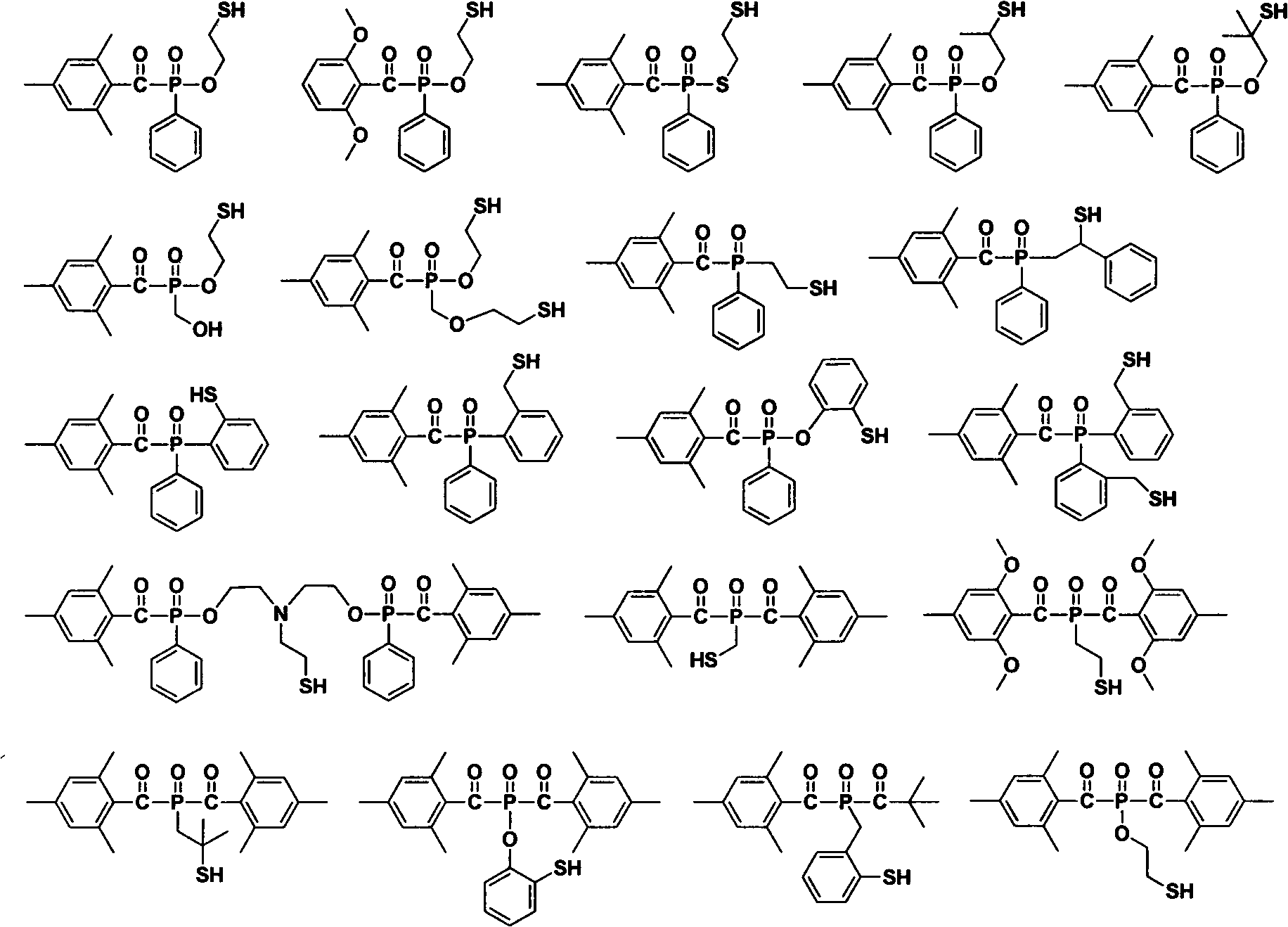
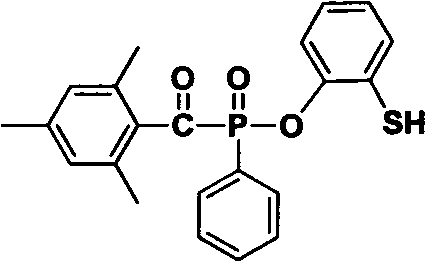
Acyl phosphine oxygen compound containing sulfydryl substituent group and photoinitiator containing compound
A technology of acyl phosphine oxide and compound, which is applied in the field of new UV light radiation free radical polymerization materials, and can solve the problems of poor surface curing effect, low activity of peroxide free radicals, and significant effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 (synthetic method of building blocks containing mercapto groups):
[0029]
[0030] Under nitrogen protection, 4.2 grams of phenylethoxyphosphine chloride (prepared with reference to literature procedure Tetrahedron, 2009, 65, 9973-9982) was placed in 60 ml of freshly distilled tetrahydrofuran solvent, and 4.4 grams of acetic acid (2-hydroxyl phenylthioester) and 3.6 milliliters of dry triethylamine, the mixture was stirred at room temperature for 4 hours and then evaporated to remove most of the solvent, the residue was diluted with 100 milliliters of dichloromethane and washed once with equal volume of water and saturated brine, and the organic phase was Dry with sodium sulfate, filter, concentrate and remove the solvent, mix the residue with 100 ml of toluene, add 4.4 g of mesitluoyl chloride, the reaction releases methyl chloride gas, react for 8 hours and filter the reaction solution with diatomaceous earth, filter the cake with a small amount of Wa...
Embodiment 2
[0032] Embodiment two (mercapto substitution synthetic method):
[0033]
[0034] The first step: under nitrogen protection, 100 ml of ethylene glycol dimethyl ether solution containing 0.05 mole of bis-tritoluoyl sodium phosphide prepared by referring to the literature procedure (WO2006 / 056541A1) was cooled to zero degree, and 4.4 g of Propylene oxide and 50 ml of anhydrous N,N-dimethylformamide were sealed and heated at 60°C for 2 hours. The reaction solution was quenched with 8 milliliters of saturated ammonium chloride aqueous solution and then concentrated under reduced pressure. The residual yellow viscous liquid was mixed with 80 milliliters of toluene, and 25 milliliters of an aqueous solution with a pH of 2-3 was added, and then 7.5 milliliters of H 2 o 2 30% aqueous solution, after dripping, keep warm at 60 degrees Celsius for 2 hours. The reaction solution was poured into 250 ml of saturated brine, the organic phase was separated, dried over magnesium sulfate, ...
Embodiment 3
[0038] Prepare radiation curing test samples according to the following weight percentages: 30 parts: epoxy acrylate; 35 parts: polyester acrylate; 5 parts: hexanediol diacrylate; 3 parts: pentaerythritol triacrylate; 23 parts: titanium dioxide Pigment; 4 parts: Example photoinitiator compound.
[0039] Take part of the above mixture and grind it evenly, spray it on the white substrate, and form a coating of about 20 microns in the air. A 250W high-pressure mercury lamp was used as the light source to irradiate at a distance of 12 cm from the sample. Finger pressure scraping method to determine the complete curing of the coating. Compounds 1 and 2 of the above-mentioned examples both initiate the curing of the film layer completely, showing good photosensitive initiation performance.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com