Porous manganese phosphate lithium-carbon composite material and preparation method
A technology of lithium iron manganese phosphate and carbon composite material, applied in nanotechnology, structural parts, electrical components and other directions for materials and surface science, can solve the problems of high cost, complex preparation method, low volume energy density, etc. The effect of low carbon content, simple preparation method and high active substance content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1: Take 18mL 50%Mn(NO 3 ) 2 Aqueous solution, 20mL 85%H 3 PO 4 Aqueous solution, 70mL ethanol, 20mL water were mixed and stirred at 25°C for 18 hours to prepare MnPO 4 ·H 2 O material, after filtration and drying, was heat-treated in an Ar atmosphere at 600 °C for 10 h to obtain the intermediate product Mn 2 P 2 o 7 , the scanning electron micrograph (SEM) of the sample as figure 1 As shown, it can be seen that the primary particle size is about 50nm, and they are agglomerated together to form microspheres, and there are nanoholes of 5-50nm between the particles. Weigh 0.8g Mn 2 P 2 o 7 With 0.44g ferrous oxalate (FeC 2 o 4 ), 0.39g lithium hydroxide (LiOH·H 2 O), 0.28g ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ), 0.2 g of polyethylene glycol (PEG), mixed with 15 mL of ethanol for ball milling for 6 h, and then dried at 80° C. to obtain the second reaction precursor. The second reaction precursor was heat-treated at 600°C for 10 hours in an...
Embodiment 2
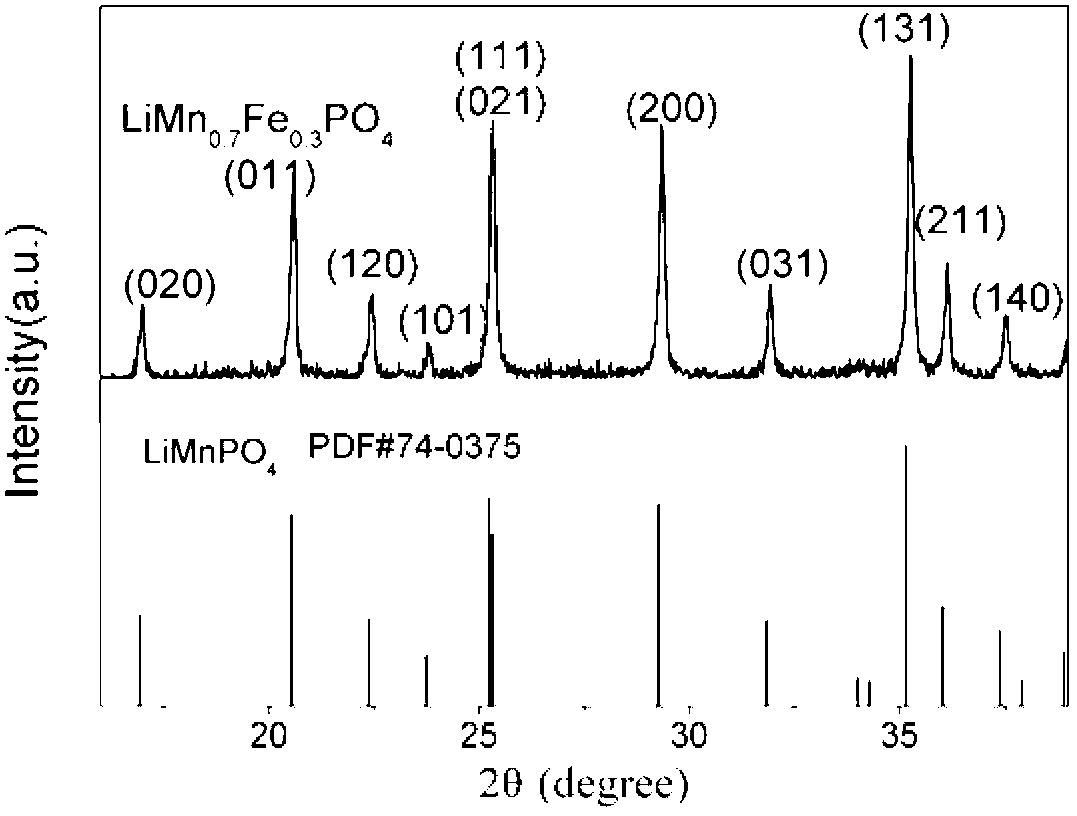
[0030] Example 2: Take 18mL 50%Mn(NO 3 ) 2 Aqueous solution, 20mL 85%H 3 PO 4 Aqueous solution, 70mL ethanol, 20mL water were mixed and stirred at 25°C for 18 hours to prepare MnPO 4 ·H 2 O material, after filtration and drying, heat treatment in air atmosphere at 600 °C for 5 h to obtain the intermediate product Mn 2 P 2 o 7 . Weigh 0.8g Mn 2 P2 o 7 With 0.44g ferrous oxalate (FeC 2 o 4 ), 0.39g lithium hydroxide (LiOH·H 2 O), 0.28g ammonium dihydrogen phosphate (NH 4 h 2 PO 4 ), 0.4 g of PEG, mixed with 15 mL of ethanol for ball milling for 6 h, and then dried at 80° C. to obtain the second reaction precursor. The second reaction precursor was heat-treated at 600°C for 10 hours in an Ar flow to obtain the final product, in which the general structural formula of the lithium manganese iron phosphate material was LiMn 0.7 Fe 0.3 PO 4 . The carbon content in the composite material is about 4wt% as determined by an elemental analyzer. By using the same meth...
Embodiment 3
[0031] Example 3: Take 18mL 50%Mn(NO 3 ) 2 Aqueous solution, 20mL 85%H 3 PO 4 Aqueous solution, 70mL ethanol, 20mL water were mixed and stirred at 25°C for 18 hours to prepare MnPO 4 ·H 2 O material, after filtration and drying, was heat-treated at 600 °C for 5 h in an Ar atmosphere to obtain the intermediate product Mn 2 P 2 o 7 . Weigh 1.42g Mn 2 P 2 o 7 With 0.4g lithium carbonate (Li 2 CO 3 ), 0.5 g of glucose, and added 15 mL of ethanol for ball milling for 6 h, then dried at 80° C. to obtain the second reaction precursor. The second reaction precursor was heat-treated at 700°C for 10 hours in an Ar flow to obtain the final product, whose general structure is LiMnPO 4 . The carbon content in the composite material is about 8wt% as determined by an elemental analyzer. By using the same method as in Example 1, the first discharge specific capacity of the positive electrode material was measured to be 30mAh / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com