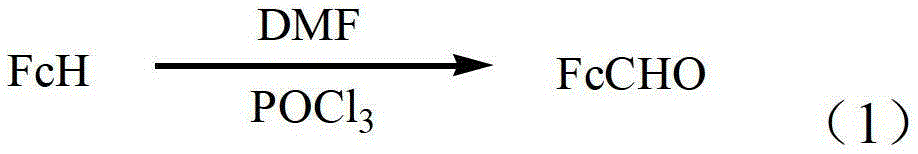
Method for preparing ferrocenecarboxaldehyde
A technology of ferrocene formaldehyde and dichloromethane, applied in chemical instruments and methods, metallocenes, organic chemistry, etc., can solve the problems of complex post-processing, low yield, long reaction time, etc., and achieve short reaction time and high yield High efficiency and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] In the first step, add 0.07mol DMF (N,N-dimethylformamide) to a dry three-necked flask, add 0.07mol phosphorus oxychloride to it at a rate of 1 drop per second at 8°C, and continue stirring for 0.5 After 2 hours, slowly add 0.01mol of ferrocene, raise the temperature to 20°C at a rate of 1°C / min and react for 2.5h to obtain a mixed solution;
[0023] In the second step, the mixed solution was cooled to room temperature, 21 mL of water was slowly added thereto, the pH of the mixed solution was adjusted to 6 with a 5% NaOH solution, and the mixed solution was extracted twice with dichloromethane, dichloromethane The dosage is 20mL each time, the organic phase obtained after two extractions is combined to obtain the extract phase, the obtained extract phase is washed with water, and then dried with anhydrous magnesium sulfate to remove the moisture in the extract phase, until the anhydrous magnesium sulfate no longer forms block, then decompressed to remove the dichloromet...
Embodiment 2
[0025] In the first step, add 0.08mol DMF to a dry three-necked flask, add 0.08mol phosphorus oxychloride to it at a rate of 2 drops per second at 9°C, continue stirring for 0.5h and then slowly add 0.01mol ferrocene, Raise the temperature to 30°C at a heating rate of 1°C / min and react for 2 hours to obtain a mixed solution;
[0026] In the second step, the mixed solution was cooled to room temperature, 24 mL of water was slowly added thereto, the pH of the mixed solution was adjusted to 7 with a KOH solution with a mass concentration of 10%, and then the mixed solution was extracted twice with dichloromethane, dichloromethane The dosage is 20mL each time, the organic phases obtained after two extractions are combined to obtain the extract phase, the obtained extract phase is washed with water, and then dried with anhydrous sodium sulfate to remove the moisture in the extract phase, until the anhydrous sodium sulfate no longer solidifies block, then decompressed to remove the ...
Embodiment 3
[0028] In the first step, add 0.09mol DMF to a dry three-necked flask, add 0.09mol phosphorus oxychloride to it at a rate of 1 drop per second at 9°C, continue stirring for 0.5h and then slowly add 0.01mol ferrocene, Raise the temperature to 40°C at a heating rate of 2°C / min and react for 1.5h to obtain a mixed solution;
[0029] In the second step, the mixed solution was cooled to room temperature, 27 mL of water was slowly added thereto, the pH of the mixed solution was adjusted to 7 with a 10% NaOH solution, and then the mixed solution was extracted twice with dichloromethane, dichloromethane The dosage is 20mL each time, the organic phase obtained after two extractions is combined to obtain the extract phase, the obtained extract phase is washed with water, and then dried with anhydrous magnesium sulfate to remove the moisture in the extract phase, until the anhydrous magnesium sulfate no longer forms block, then decompressed to remove the dichloromethane in the extract ph...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com