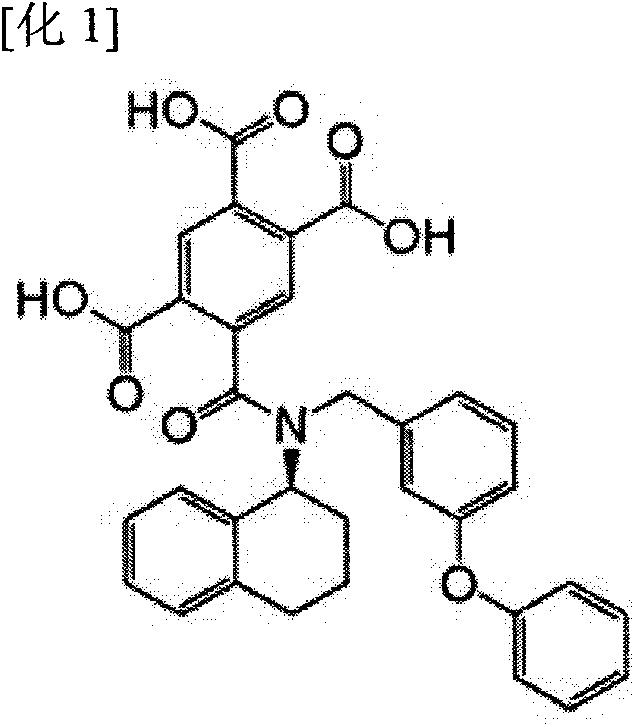
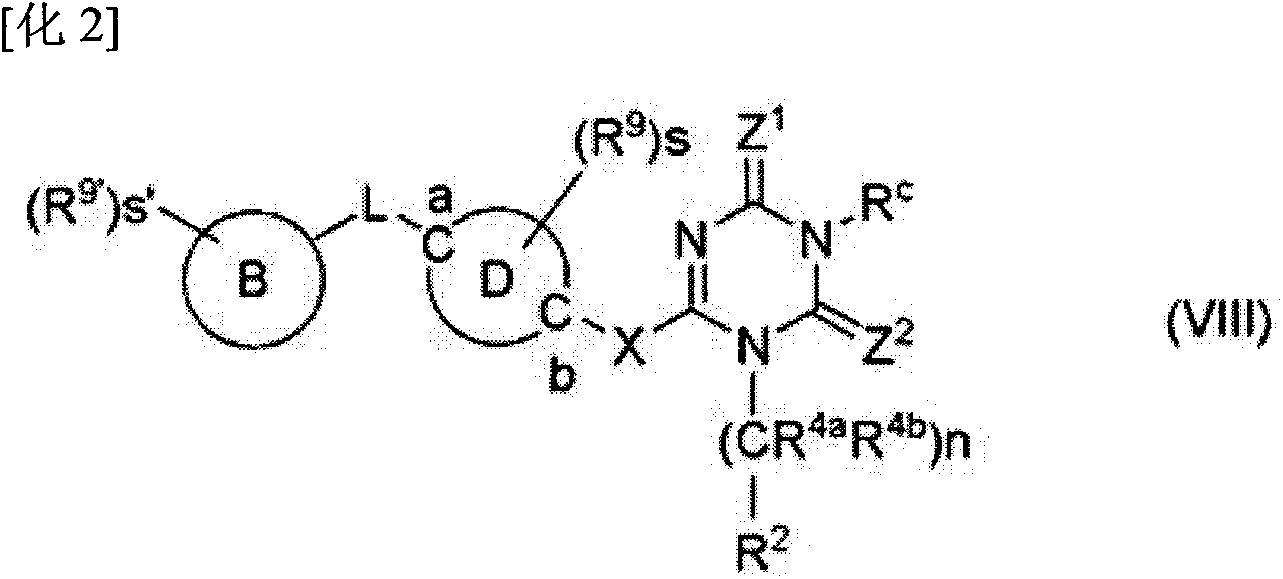
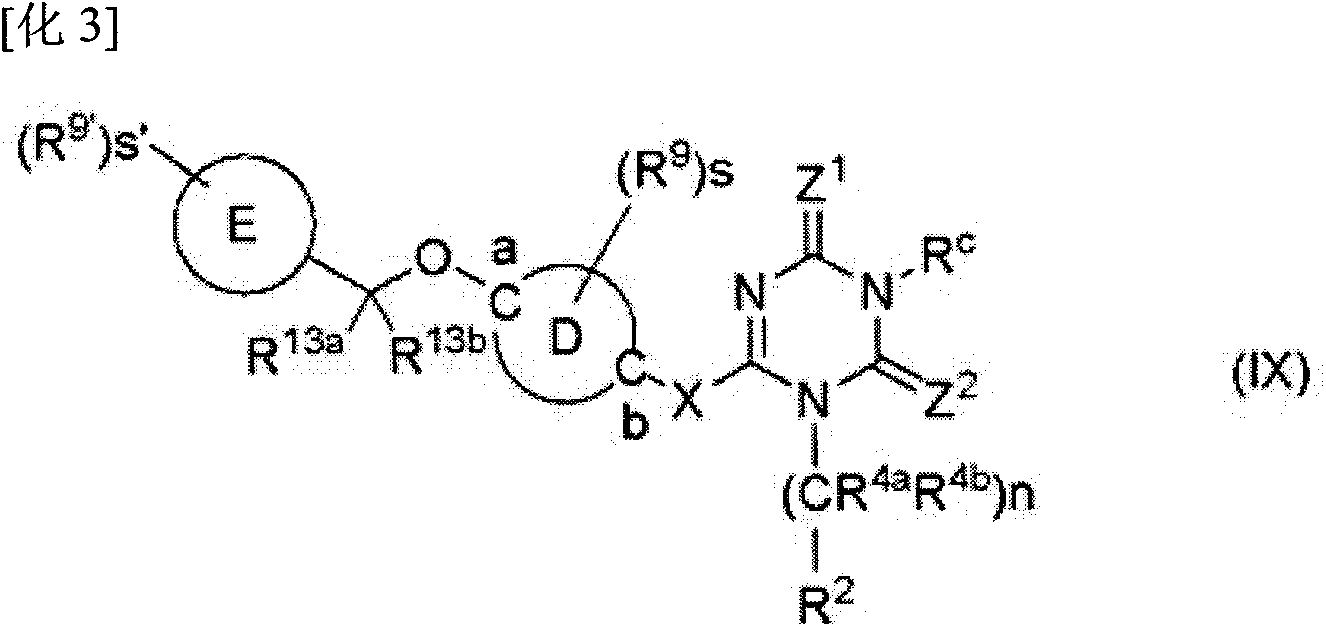
Triazine derivative and pharmaceutical compound that contains same and exhibits analgesic activity
A compound and pharmacy technology are applied in the field of triazole derivatives and pharmaceutical compositions containing the same with analgesic effect, can solve the problems such as the method of changing pyrazole group to aniline group which are not recorded or recorded, and achieve excellent analgesic effect. effect of pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[1299] Preparation of 6-(ethylthio)-1-(4-fluorobenzyl)-3-isopropyl-1,3,5-triazine-2,4(1H,3H)-dione
[1300]
[1301] Add isopropyl isocyanate (8.2mL, 84mmol) and DBU (12.6mL, 84mmol) to the mixture of S-ethylthiourea hydrobromide (14.8g, 80mmol) and DMF (75mL) under ice cooling ), stirred for 6 hours under ice cooling. Under ice cooling, 1,1'-carbonyldiimidazole (15.57 g, 96 mmol) and DBU (18.0 mL, 120 mmol) were added to the reaction solution, and the mixture was further stirred for 2 hours. After the reaction, 2 mol / L hydrochloric acid (240 mL) was added over about 50 minutes under ice cooling, and the precipitated solid was collected by filtration. The obtained solid was dissolved in ethyl acetate, dried over anhydrous magnesium sulfate, and then concentrated under reduced pressure to obtain 6-(ethylthio)-3-isopropyl-1,3,5-triazine as a light brown solid -2,4(1H,3H)-dione (12.29g, yield: 71%).
[1302] 1H-NMR (δppm TMS / DMSO-d6): 1.27 (6H, t, J=7.2 Hz)), 1.33 (6H, d, J=6.9 Hz)...
Embodiment 2
[1306] 1-(4-Fluorobenzyl)-6-(4-isopropoxyphenylamino)-3-isopropyl-1,3,5-triazine-2,4(1H,3H)-dione (I-0133) Preparation
[1307]
[1308] Under microwave irradiation, at 230°C, 6-(ethylthio)-1-(4-fluorobenzyl)-3-isopropyl-1,3,5-triazine-2,4(1H, A mixture of 3H)-dione (0.323 g, 1 mmol) and 4-isopropoxyaniline (0.907 g, 6 mmol) and 1-methyl-2-pyrrolidone (1 mL) was stirred for 30 minutes. After the reaction, water (100 mL) was added, and extraction was performed with ethyl acetate (100 mL). After drying the extract with anhydrous sodium sulfate, it was concentrated under reduced pressure, and the obtained residue was purified by silica gel column chromatography (ethyl acetate / hexane). The obtained target product was precipitated in ethyl acetate and hexane to obtain 1-(4-fluorobenzyl)-6-(4-isopropoxyphenylamino)-3-isopropyl as a colorless solid -1,3,5-triazine-2,4(1H,3H)-dione (0.21g, yield: 51%).
[1309] Melting point: 176-177°C
[1310] 1H-NMR(δppm TMS / CDCl 3 ): 1.34 (6H, d, J =...
Embodiment 3
[1312] 6-(3-Chloro-4-isopropoxyphenylamino)-1-(4-chlorobenzyl)-3-isopropyl-1,3,5-triazine-2,4(1H,3H )-Diketone preparation (I-0228)
[1313]
[1314] At 90 ℃, 1-(4-chlorobenzyl)-6-(ethylsulfanyl)-3-isopropyl-1,3,5-triazine-2,4(1H,3H)-di A mixture of ketone (0.60 g, 1.78 mmol), 3-chloro-4-isopropoxyaniline (0.99 g, 5.3 mmol) and acetic acid (10 mL) was stirred for 6 hours. After the reaction, the reaction solution was added to a saturated aqueous sodium hydrogen carbonate solution (100 mL), and extracted with ethyl acetate (100 mL). The extract was washed with saturated aqueous sodium bicarbonate (100 mL) and saturated brine (100 mL), dried over anhydrous sodium sulfate, and then concentrated under reduced pressure. The obtained residue was subjected to silica gel column chromatography (ethyl acetate / hexane) purification. The obtained target product was precipitated in diethyl ether and hexane to obtain 6-(3-chloro-4-isopropoxyphenylamino)-1-(4-chlorobenzyl)-3- as a colorless s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com