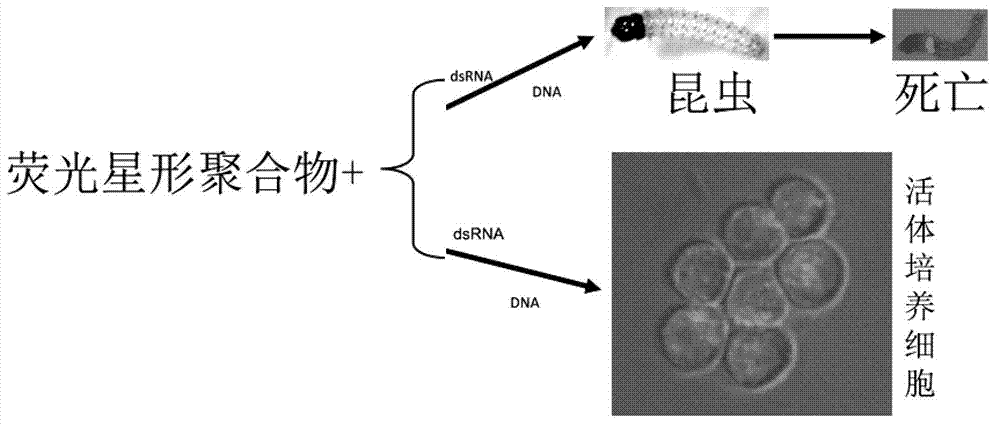
Fluorescent star-like polymer as well as preparation method and application thereof
A technology of star-shaped polymers and fluorescence, applied in other methods of inserting foreign genetic materials, chemical instruments and methods, luminescent materials, etc., can solve problems such as poor water solubility and affect applications, and achieve good biocompatibility and good applications value, low cytotoxic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Synthesis of star-shaped PAEMA (polyaminoethyl methacrylate) using ATRP polymerization method
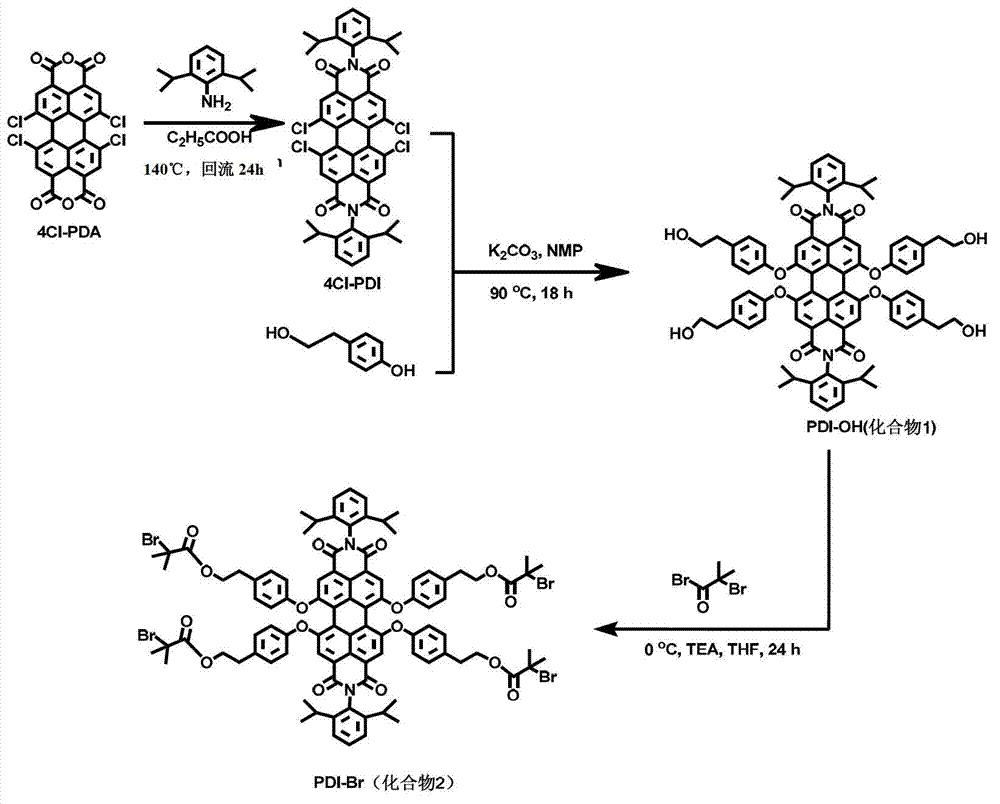
[0045] 1.1 Synthesis of PDI-OH (compound 1)
[0046] 4Cl-PDI (1.70g, 2mmol), p-hydroxyphenethyl alcohol (2.76g, 20mmol) and K 2 CO 3 (1.38g, 10mmol) was added to a 250mL three-necked flask, and 80mL of NMP (N-methylpyrrolidone) was added to completely dissolve the reactant. Then pass nitrogen gas for 15 minutes, remove the air, put the flask into a 90°C oil bath to start the reaction, monitor the reaction degree by thin-layer chromatography, and stop the reaction after 24 hours. The reactant was precipitated in a mixed solution of water / methanol / concentrated hydrochloric acid (v / v / v=5:40:2), and NMP was removed by suction filtration to obtain a crude product. Using ethyl acetate as the eluent, it was separated by column chromatography to finally obtain 2.20 g of the product (compound 1), with a yield of 88%.
[0047]
[0048] Compound 1
[0049] 1.2 Synthesi...
Embodiment 2
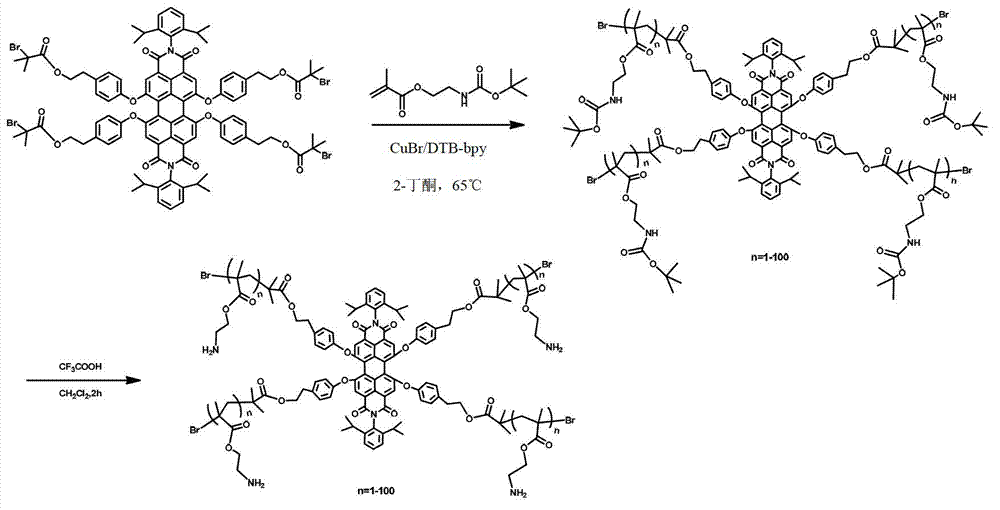
[0078] Synthesis of embodiment 2 fluorescent star polymer 3 (n=50)
[0079]Add initiator compound 2 (17.6mg, 9.6μmol), catalyst CuBr (5.5mg, 38.4μmol), monomer Boc-AEMA (880mg, 3.84mmol) into a 25mL reaction tube, dissolve with 2mL 2-butanone, and process 3 times The freezing pumping process achieves the purpose of deoxygenation. The ligand DTB-bipy (20.6 mg, 76.8 μmol) is added under the protection of an inert gas, and then sealed with a rubber stopper. After stirring CuBr and the ligand are completely complexed, the reaction tube is Add to 65 ° C oil bath to start the reaction. After 50 minutes of reaction, the reactant passed through a neutral alumina column to remove the catalyst, and then precipitated three times in a mixed solvent of methanol and water (v:v=1:1), dried in vacuum to constant weight, and obtained 430 mg fluorescent star polymerization Object 3 (chain length n=50).
[0080] Deprotection of fluorescent star polymer 3 (n = 50): Take 400 mg fluorescent star ...
Embodiment 3
[0081] Synthesis of embodiment 3 fluorescent star polymer 3 (n=1)
[0082] Add initiator compound 2 (35.2 mg, 19.2 μmol), catalyst CuBr (11 mg, 76.8 μmol), monomer Boc-AEMA (880 mg, 3.84 mmol) into a 25 mL reaction tube, dissolve with 2 mL of 2-butanone, and freeze 3 times The pumping process achieves the purpose of deoxygenation. The ligand DTB-bipy (20.6 mg, 76.8 μmol) is added under the protection of an inert gas, and then sealed with a rubber stopper. After stirring CuBr and the ligand are completely complexed, the reaction tube is added The reaction was started in an oil bath at 65°C. After 5 minutes of reaction, the reactant was passed through a neutral alumina column to remove the catalyst, and then precipitated in a mixed solvent of methanol and water (v:v=1:1) for 3 times, and dried in vacuum to constant weight to obtain 52 mg fluorescent star polymerization Object 3 (chain length n=1).
[0083] Deprotection of fluorescent star polymer 3 (n = 1): Take 50 mg fluoresc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com