Preparation method of tin-cobalt alloy cathode material of lithium ion battery
A technology for lithium-ion batteries and negative electrode materials, which is applied to battery electrodes, circuits, electrical components, etc., can solve problems such as poor cycle performance, and achieve the effects of high tap density, low energy consumption, and less time consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
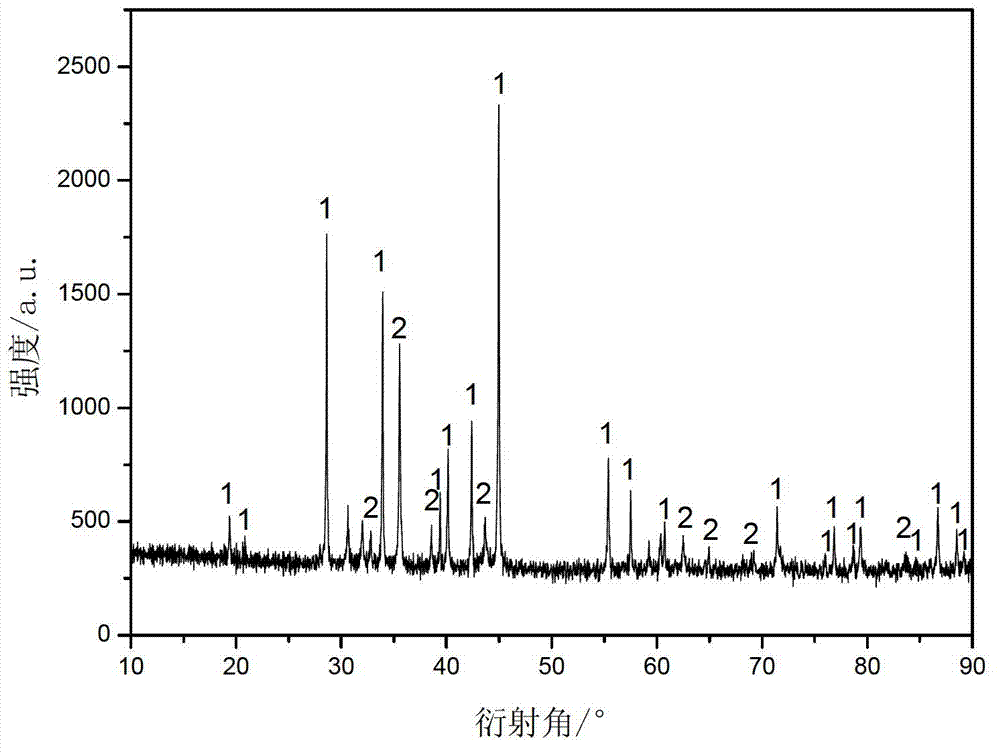
Embodiment 1
[0022] Add an appropriate amount of sulfuric acid to 180 mL of deionized water to adjust the pH to 0.5, then add 40 mg of ascorbic acid, 25 mg of polyvinylpyrrolidone, and finally 6 g of stannous sulfate and 7.8 g of cobalt sulfate to the deionized water to obtain solution A. Add 7 g of sodium carbonate to 80 ml of deionized water to obtain solution B. At room temperature, while stirring, solution B was added dropwise to solution A at the same time to obtain solution C, and stirred for 3 hours after the reaction. The solution C was suction filtered and washed with a large amount of deionized water until there was no sulfate in the filtrate. After suction filtration, the filter cake was dispersed in ethanol by means of stirring and ultrasonic treatment, and then the ethanol was filtered off by suction, and the filter cake was dried in air at 80° C. for 6 hours to obtain a precursor. Weigh 2g of the precursor, 2g of polyvinylpyrrolidone, and ethanol as the solvent, and evaporat...
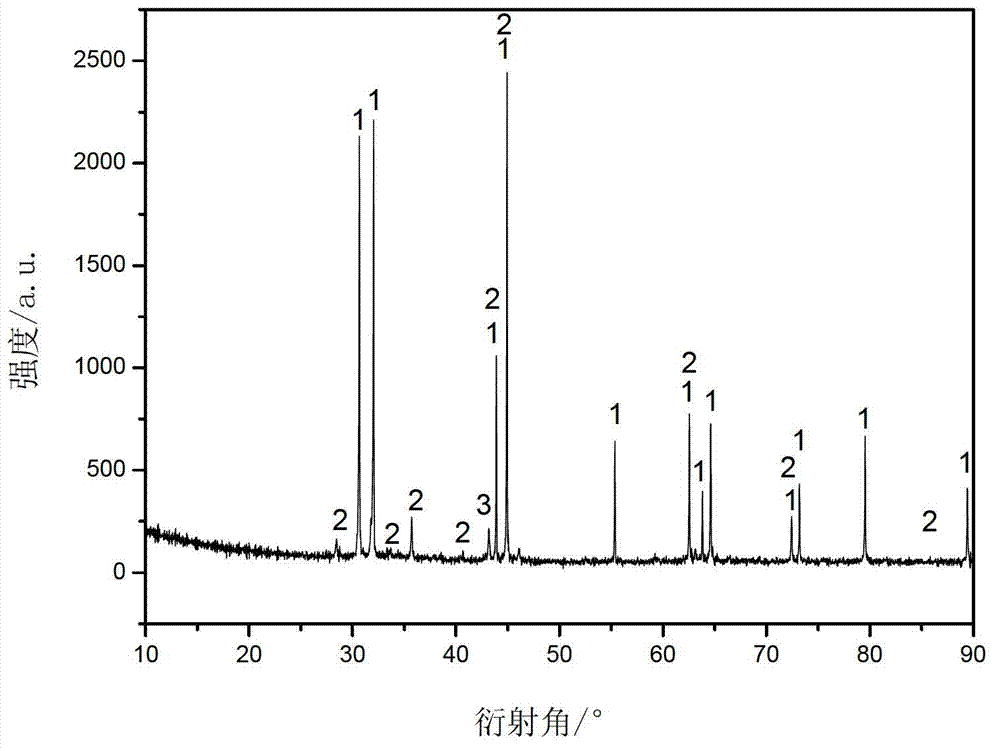
Embodiment 2
[0026]Add an appropriate amount of sulfuric acid to 200mL of deionized water to adjust the pH to 1, then add 45mg of ascorbic acid, 30mg of polyvinylpyrrolidone to the deionized water, and finally add 9g of stannous sulfate and 4g of cobalt sulfate to obtain solution A. Add 11 g of potassium carbonate to 80 ml of deionized water to obtain solution B. At room temperature, while stirring, solution B was added dropwise to solution A at the same time to obtain solution C, and stirred for 2 hours after the reaction. The solution C was suction filtered and washed with a large amount of deionized water until there was no sulfate in the filtrate. After suction filtration, the filter cake was dispersed in ethanol by means of stirring and ultrasonic treatment, and then the ethanol was filtered off with suction, and the filter cake was dried in air at 80° C. for 12 hours to obtain a precursor. Weigh 2g of the precursor, transfer 2g of glucose to a hydrothermal kettle for hydrothermal tr...
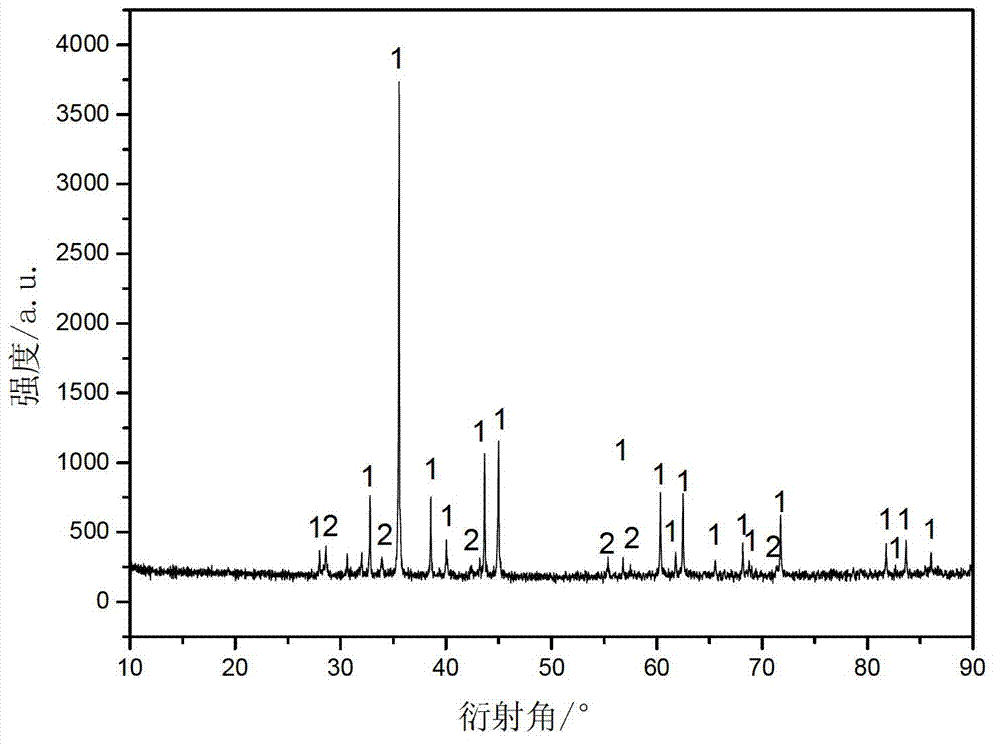
Embodiment 3
[0029] Add an appropriate amount of sulfuric acid to 200mL of deionized water to adjust the pH to 2, then add 45mg of ascorbic acid, 30mg of polyvinylpyrrolidone to the deionized water, and finally add 10g of stannous sulfate and 6g of cobalt sulfate to obtain solution A. Add 8 g of potassium hydroxide to 80 ml of deionized water to obtain solution B. At room temperature, while stirring, solution B was added dropwise to solution A at the same time to obtain solution C, and stirred for 2 hours after the reaction. The solution C was suction filtered and washed with a large amount of deionized water until there was no sulfate in the filtrate. After suction filtration, the filter cake was dispersed in ethanol by means of stirring and ultrasonic treatment, and then the ethanol was filtered off with suction, and the filter cake was dried in air at 80° C. for 12 hours to obtain a precursor. Weigh 2g of the precursor, 2g of stearic acid, and ethanol as the solvent, and evaporate the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com