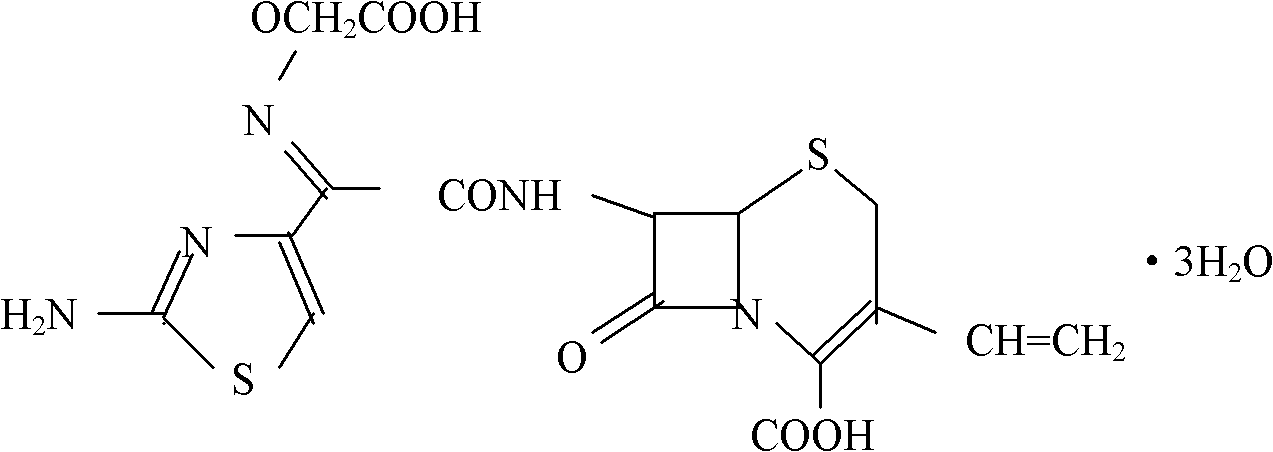
Cefixime orally disintegrating tablet and preparation thereof
A technology of orally disintegrating tablets and cefixime, which is applied to pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., to achieve the effects of increased absorption, less stimulating effect, and improved compliance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
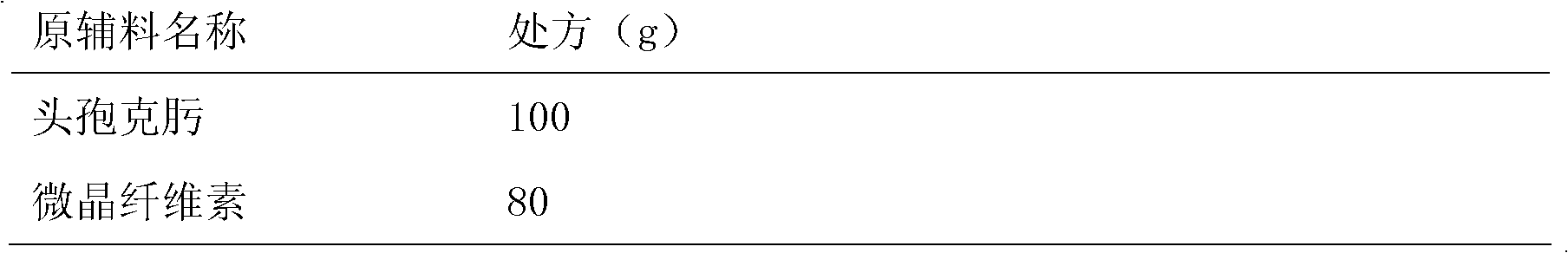
[0022]
[0023]
[0024] preparation:
[0025] (1) Take the raw materials of each component in the prescription and pass through a 40-mesh sieve respectively, except for cefixime, and dry them at 60°C for later use;
[0026] (2) Step (1) Mix cefixime and other spare parts evenly, and sieve with 40 mesh.
[0027] (3) Determination of intermediate content (cefixime 334 ~ 340mg / g) and moisture (7.6% ~ 7.8%), calculate the theoretical tablet (0.271g, equivalent to cefixime 100mg), compressed into 1000 tablets, each Sheet thickness: 4.25~4.75mm, hardness: 2.0kg / cm 2 ~4.0kg / cm 2 .
[0028] Die of tablet press (ZP-10 type): ф10mm shallow arc punch.
Embodiment 2
[0030] Recipe and preparation are with embodiment 1.
Embodiment 3
[0032] Recipe and preparation are with embodiment 1.
[0033] The disintegration times of the three batches of trial samples (Example 1, 2, 3) were all within 40 seconds, meeting the preparation requirements for orally disintegrating tablets.
[0034] Dissolution rate: adopt the device of dissolution test method (the first method of appendix XC of Chinese Pharmacopoeia 2005 edition). Using 900ml of water as a solvent, the rotation speed is 100 revolutions per minute, and the dissolution rate of three batches of trial samples (batch numbers: 20090101T, 20090102T, 20090103T) exceeds 80% in 10 minutes.
[0035] The orally disintegrating tablet of the present invention (specification 0.1g, Example 1) and the ordinary tablet on the market (Guangzhou Baiyunshan Pharmaceutical General Factory, specification 0.1g, batch number: 5080001) have carried out comparative research on the difference in in vitro dissolution, which is the formulation of quality standards. And provide a basis f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com