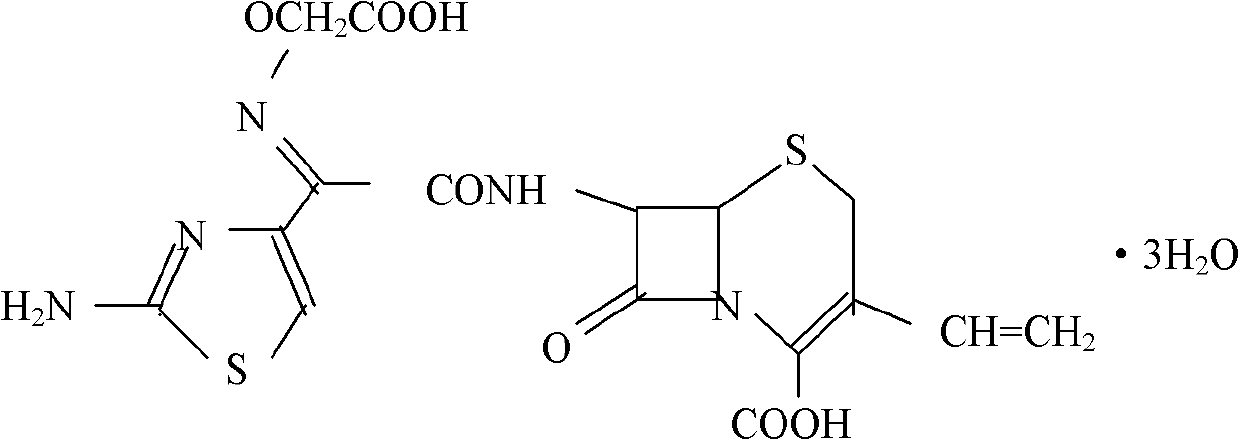
Cefixime orally disintegrating tablet and preparation thereof
A technology of orally disintegrating tablets and cefixime, which is applied in the direction of pharmaceutical formulas, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effects of fast absorption, increased absorption area, and fine drug particles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
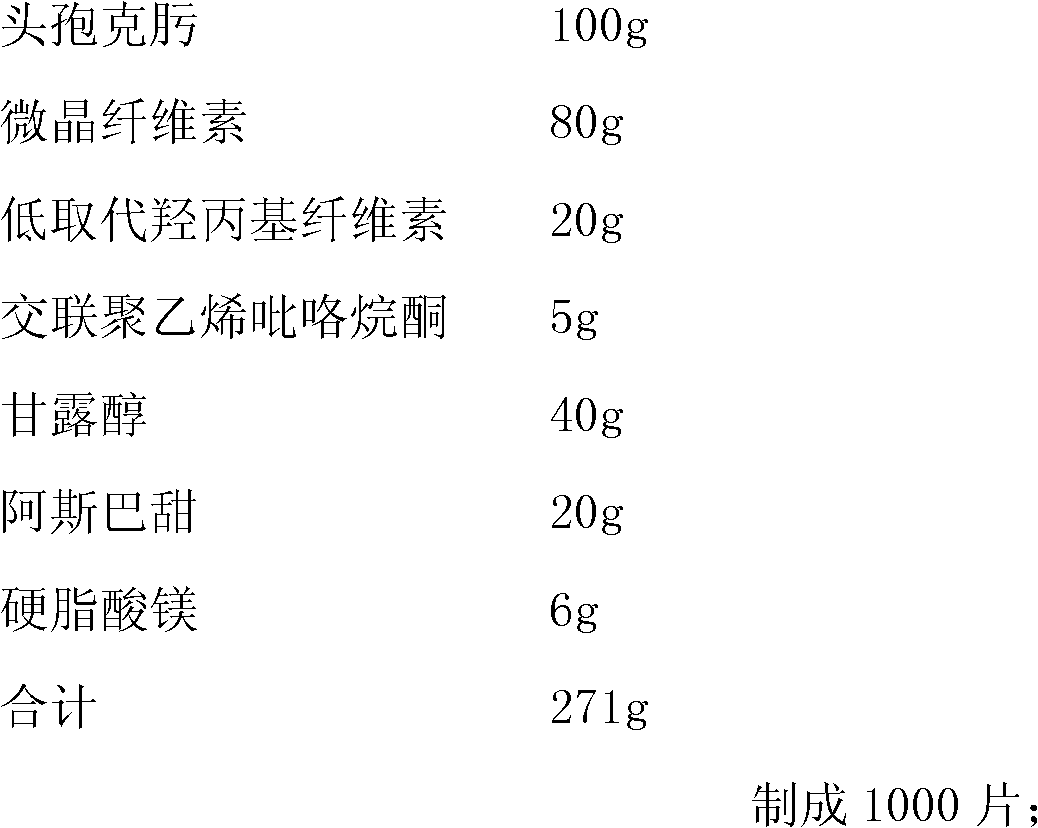
[0022]
[0023]
[0024] preparation:
[0025] (1) Take the raw materials of each component in the prescription and pass through a 40-mesh sieve respectively, except for cefixime, and dry them at 60°C for later use;
[0026] (2) Step (1) Mix cefixime and other spare parts evenly, and sieve with 40 mesh.
[0027] (3) Determination of intermediate content (cefixime 334 ~ 340mg / g) and moisture (7.6% ~ 7.8%), calculate the theoretical tablet (0.271g, equivalent to cefixime 100mg), compressed into 1000 tablets, each Sheet thickness: 4.25~4.75mm, hardness: 2.0kg / cm 2 ~4.0kg / cm 2 .
[0028] Die of tablet press (ZP-10 type): ф10mm shallow arc punch.
Embodiment 2
[0030] Recipe and preparation are with embodiment 1.
Embodiment 3
[0032] Recipe and preparation are with embodiment 1.
[0033] The disintegration times of the three batches of trial samples (Example 1, 2, 3) were all within 40 seconds, meeting the preparation requirements for orally disintegrating tablets.
[0034] Dissolution rate: adopt the device of dissolution test method (the first method of appendix XC of Chinese Pharmacopoeia 2005 edition). Using 900ml of water as a solvent, the rotation speed is 100 revolutions per minute, and the dissolution rate of three batches of trial samples (batch numbers: 20090101T, 20090102T, 20090103T) exceeds 80% in 10 minutes.
[0035] The orally disintegrating tablet of the present invention (specification 0.1g, Example 1) and the ordinary tablet on the market (Guangzhou Baiyunshan Pharmaceutical General Factory, specification 0.1g, batch number: 5080001) have carried out comparative research on the difference in in vitro dissolution, which is the formulation of quality standards. And provide a basis f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com