Method and kit for detecting drug resistance gene mutant type of tuberculous bacillus (TB)
A technology of Mycobacterium tuberculosis and drug resistance gene, applied in the field of molecular biology, can solve the problems of low throughput, low resolution, high background signal, etc., and achieve the effect of reducing usage, simple and stable reagent consumables
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] The design of embodiment 1 primer
[0060] (1) PCR amplification primers:
[0061] According to the drug-resistant gene mutations of the five TB drugs selected to be detected, design specific amplification primers for each drug-resistant gene, and the amplification primers can amplify a DNA sequence including the mutation site. The amplification primer has at least 15 bases at the 3' end that completely match the gene sequence it is targeting, and has a tag sequence of 10 bases (acgttggatg) at the 5' end to distinguish the amplification primer from the extension primer.
[0062] (2) Mass spectrometry extension primer:
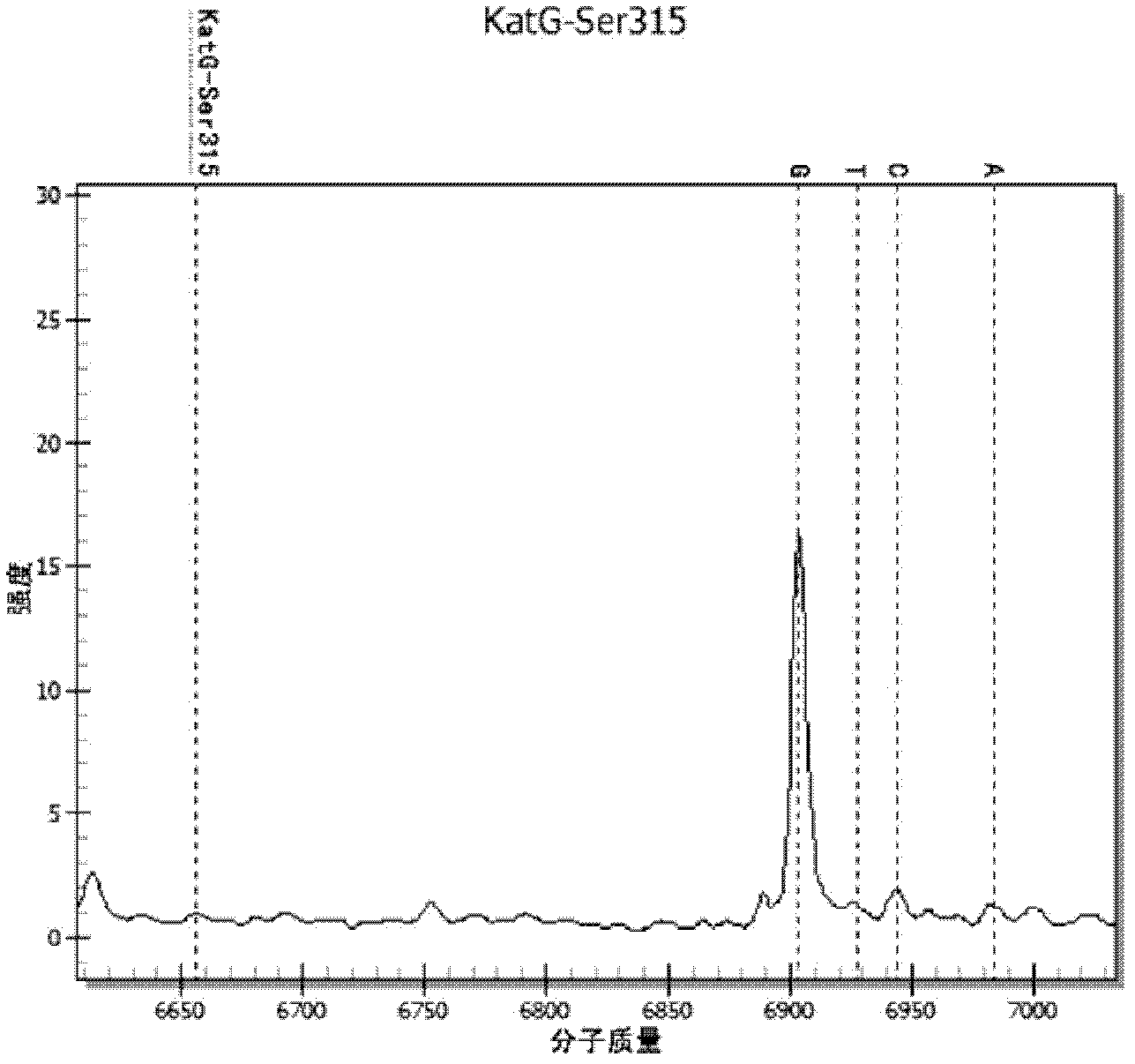
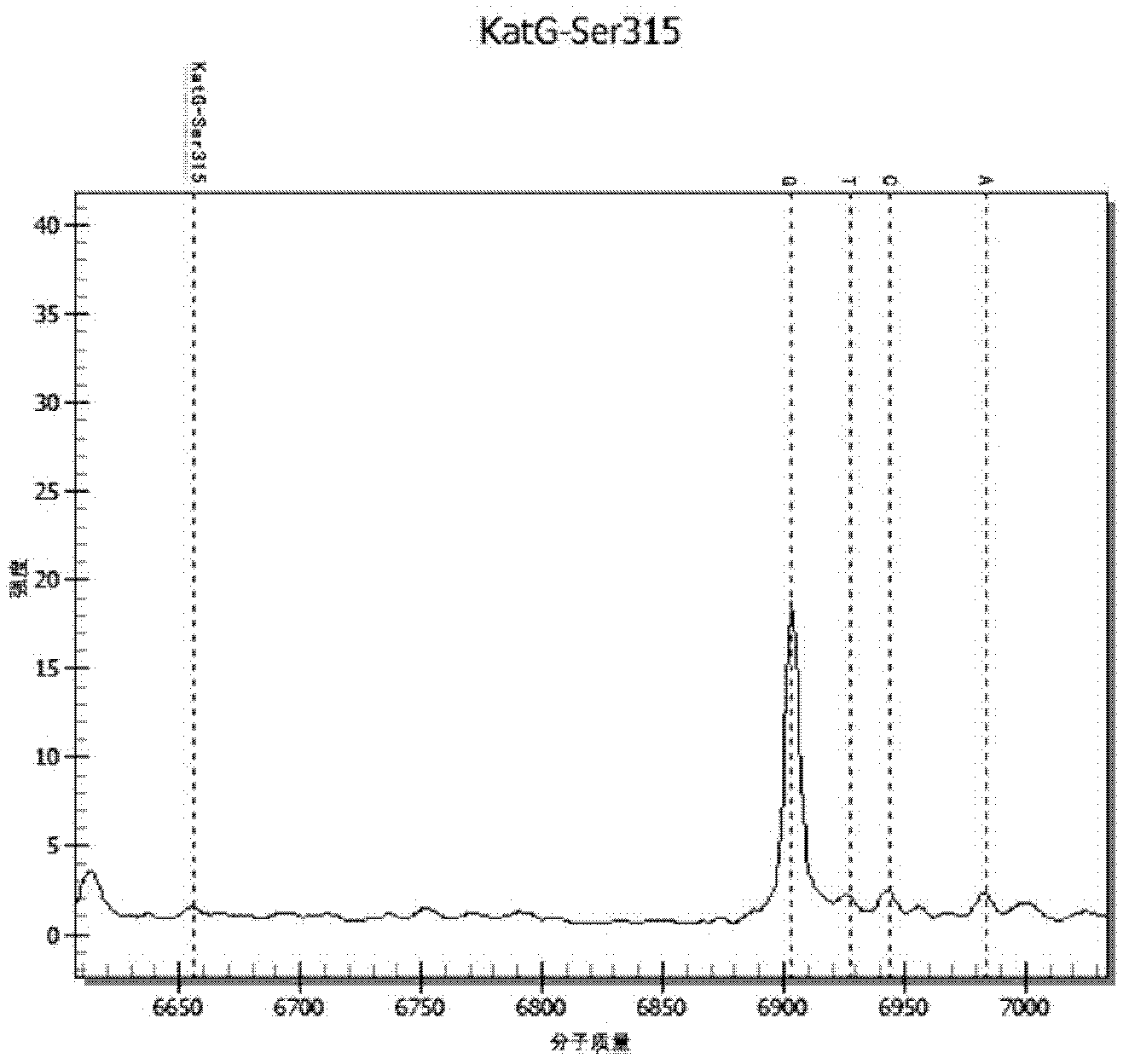
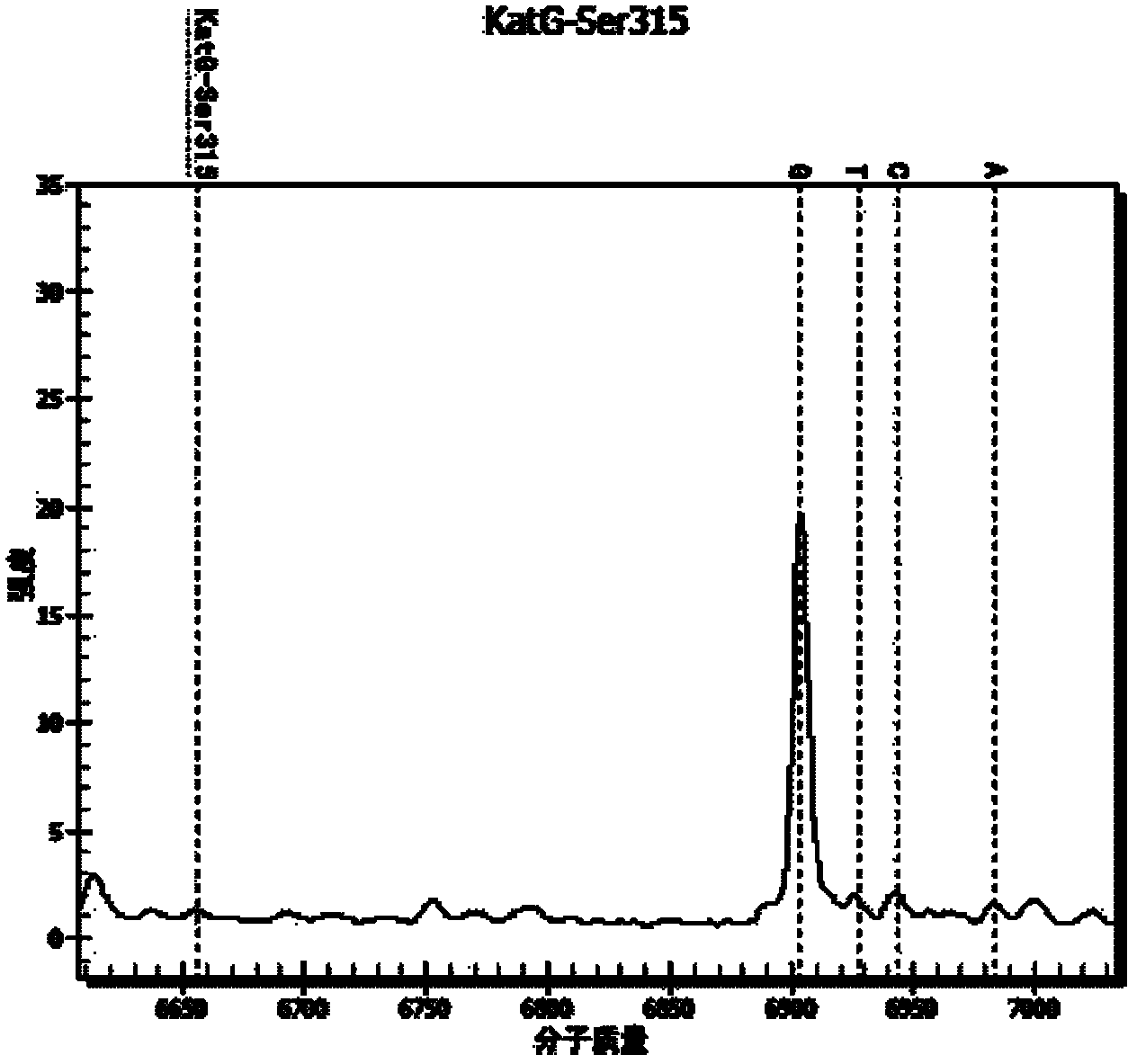
[0063] Design an extension primer, the length of the extension primer is 17-28 bases, and its 3' end is located at the last base of the drug-resistant mutation site, the extension primer only extends one base when the extension reaction occurs, and the extension The base is the resistance mutation site. Wherein, the molecular weight difference between...
Embodiment 2
[0076] Preparation of detection template
[0077] The cultured Mycobacterium tuberculosis standard strain CMCC 93009 strain and INH drug-resistant clinical isolate were counted and diluted to 10 5 Bacteria / ml, 10 4 Bacteria / ml, 10 3 Bacteria / ml concentration gradient, each concentration is prepared into a mixture of different proportions: (1) 100% CMCC 93009 bacterial strain, (2) 5% INH drug-resistant clinical isolates added to 93009 bacterial strain, (3) 93009 bacterial strain Add 25% INH resistant clinical isolates, (4) 93009 strains add 50% INH resistant clinical isolates, (5) 93009 strains add 75% INH resistant clinical isolates, (6) 100% INH resistant clinical isolates. DNA was extracted from the above-mentioned mixtures of different concentrations and different ratios (using Tiangen Bacterial DNA Kit for extraction, and the operation method was extracted according to the instructions of the kit).
Embodiment 3
[0078] The detection method of the drug-resistant gene mutant type of embodiment 3TB medicine
[0079] (1) Using the DNA extracted in Example 2 as a template, use the PCR amplification primers in Example 1 to amplify by PCR to obtain the target sequence amplification product. See Table 4 for the PCR amplification reaction system. Among them, all reagents were purchased from Sequenom Company.
[0080] Table 4: PCR amplification reaction system
[0081]
[0082]
[0083] The PCR reaction conditions were 94°C for 15 minutes; denaturation at 94°C for 20 seconds, annealing at 56°C for 30 seconds, extension at 72°C for 1 minute, and a total of 45 cycles of amplification; finally, extension at 72°C for 3 minutes. In this example, the DNA extracted in Example 2 was used as the DNA template for PCR amplification. At the same time, sterile double distilled water was used as a negative control. The control sample and the test sample were reacted and tested according to the same...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com