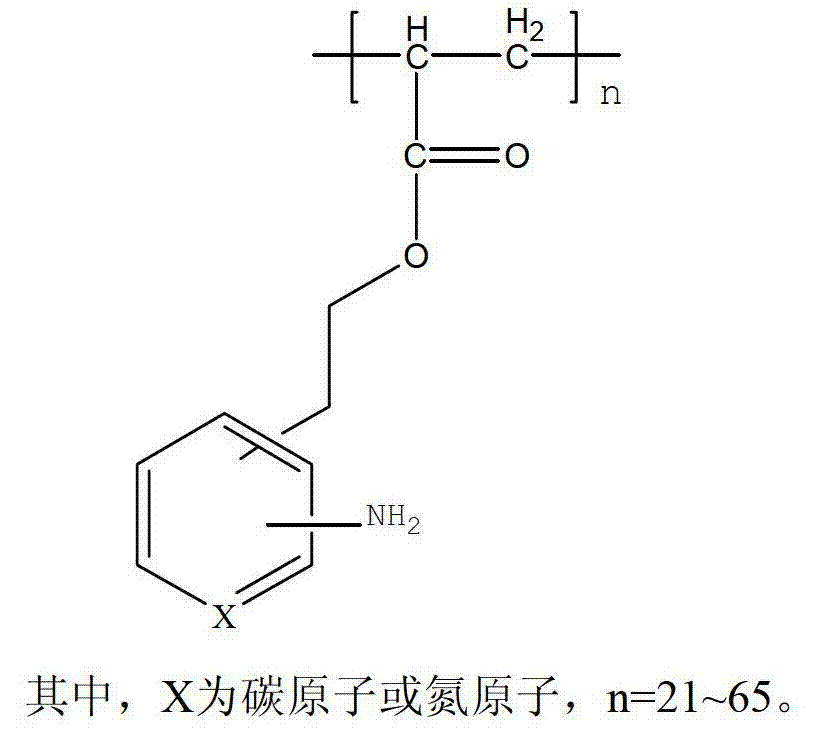
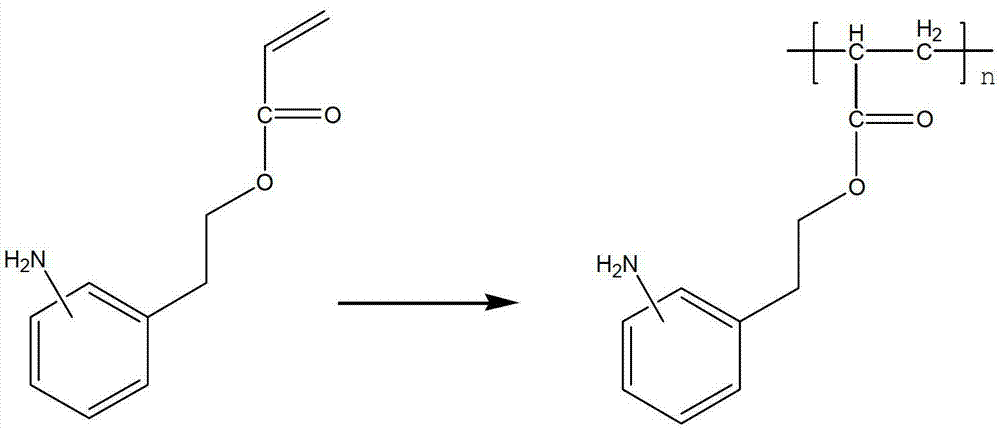
Amino arene structure unit-containing polyacrylate functionalized polymer and preparation method thereof
A technology of structural units and aminoaromatics, applied in the direction of organic chemistry, can solve the problems of high yield, low product cost, high price, etc., and achieve the effect of high yield, less waste water, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 400 grams of isopropanol, 100 grams of deionized water, and 95.5 grams (0.5 moles) of 2-(2-aminobenzene) ethanol acrylate into a 1000 ml stainless steel reactor; heat to an internal temperature of 65-75 degrees, drop 1.6 A solution of 1 gram of octyl peroxide and 10 grams of isopropanol, after dropping, the temperature was raised to 85-95°C, and the reaction was carried out for 5 hours. Alkyl mercaptan, heat preservation reaction for 2 hours; filter while hot, remove a small amount of high molecular weight polymer; filtrate is cooled to room temperature, filter, dry, obtain (formula 1) poly 2-(2-aminophenyl) ethanol acrylate 77.4 grams , the appearance is off-white solid particles, the number average molecular weight ranges from 4000 to 12000, swells in hot water, soluble in hot alcohol, and the yield is 81.0%.
Embodiment 2
[0041] Use 95.5 grams of 2-(3-aminophenyl)ethanol acrylate as a monomer to replace 2-(2-aminophenyl)ethanol acrylate in Example 1 for free radical polymerization. The specific process of the reaction is the same as in Example 1. The material yield is shown in Table 1.
Embodiment 3
[0043] Use 95.5 grams of 2-(4-aminophenyl)ethanol acrylate as a monomer to replace 2-(2-aminophenyl)ethanol acrylate in Example 1 for free radical polymerization. The specific process of the reaction is the same as in Example 1. The material yield is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com