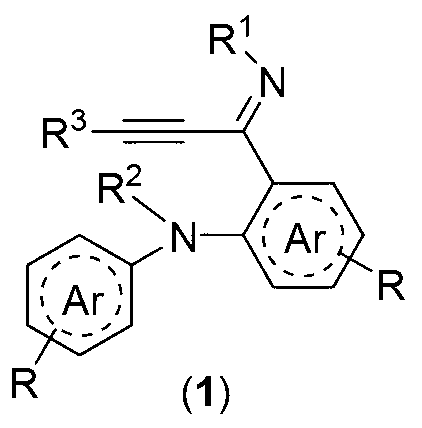
Alkynyl imine derivative
A hydrocarbon-based and aryl-based technology, applied in the field of alkynyl imine derivatives, can solve the problems of unavailable raw materials, poor regioselectivity, and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
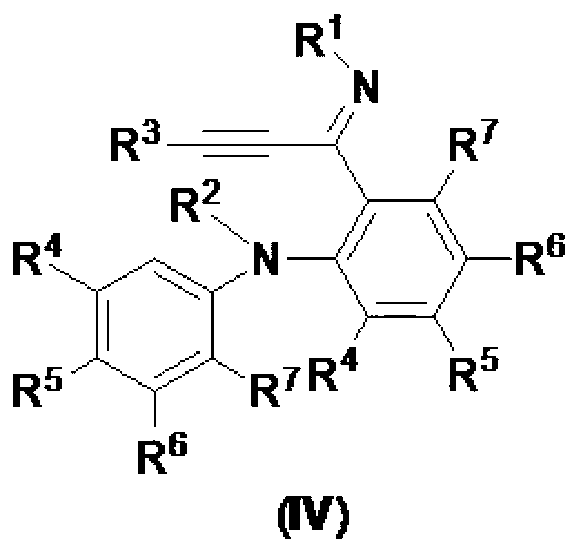
[0093] Embodiment 3——compound shown in preparation formula IVc (R 1 =R 2 = i Pr, R 3 =4-HCC-Ph, R 4 =R 5 =R 6 =R 7 =H):
[0094]
[0095] IVc
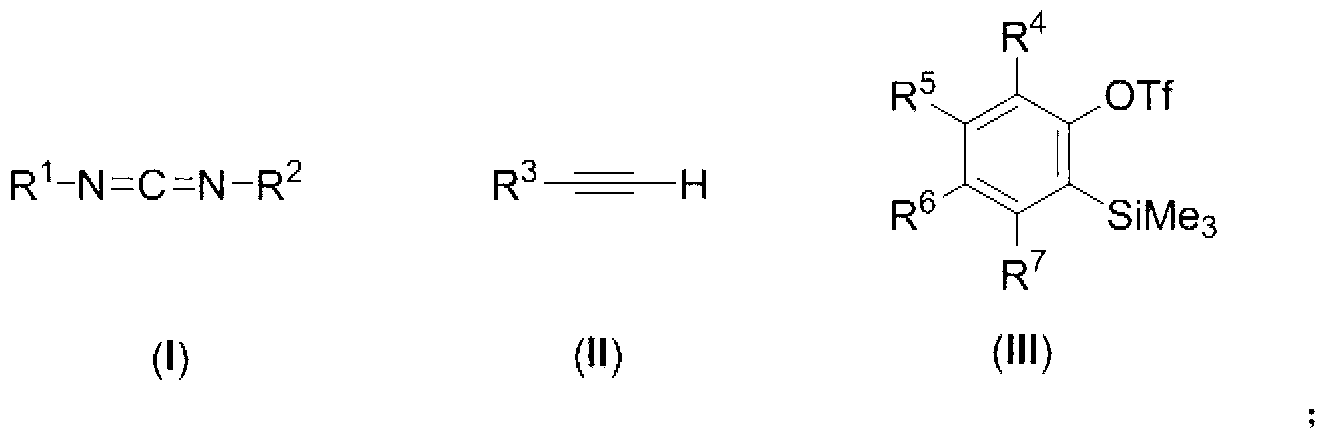
[0096] Under nitrogen protection, add 2 mmol o-trimethylsilylphenol triflate, 1 mmol N,N'-diisopropylcarbodiimide and 1 mmol 1,4-diethynylbenzene to a 25 mL reaction tube, add 3 mL THF dissolved. Then add 2.2mmol of anhydrous potassium fluoride and 2.2mmol of 18-crown-6, and react at room temperature (25°C) for 9 hours. The color of the reaction solution changed from colorless to yellow. The reaction solution was concentrated, decolorized and separated on a silica gel column, and a mixed solvent of petroleum ether: ethyl acetate: dichloromethane = 40:1:1 was used as an eluent to obtain N-{1-[2-(N-isopropyl- N-phenyl)phenyl]-3-(4-ethynylphenyl)-2-propynylalkenyl}-isopropylamine 316 mg (purity>98%, yellow oil), isolated yield 78%. The NMR data of this compound are as follows: 1 HNMR (400MHz, CD 2 Cl 2 , Me 4 Si): δ1.1...
Embodiment 4
[0097] Embodiment 4——compound shown in preparation formula IVd (R 1 =R 2 = i Pr, R 3 =4-NC-Ph,R 4 =R 5 =R 6 =R 7 =H):
[0098]
[0099] IVd
[0100] Under nitrogen protection, add 2mmol o-trimethylsilylphenol trifluoromethanesulfonate, 1mmol N,N'-diisopropylcarbodiimide and 1mmol p-cyanophenylacetylene to a 25mL reaction tube, add 3mL tetrahydrofuran to dissolve . Then add 2.2mmol of anhydrous potassium fluoride and 2.2mmol of 18-crown-6, and react at room temperature (25°C) for 9 hours. The color of the reaction solution changed from colorless to yellow. The reaction solution was concentrated, decolorized and separated on a silica gel column, and a mixed solvent of petroleum ether: ethyl acetate: dichloromethane = 40:1:1 was used as an eluent to obtain N-{1-[2-(N-isopropyl- N-phenyl)phenyl]-3-(4-cyanophenyl)-2-propynylalkenyl}-isopropylamine 320 mg (purity>98%, yellow oil), isolated yield 79%. The NMR data of this compound are as follows: 1 HNMR (400MHz, CD 2...
Embodiment 5
[0101] Embodiment 5——compound shown in preparation formula IVe (R 1 =R 2 = i Pr, R 3 =4-PhCO-Ph,R 4 =R 5 =R 6 =R 7 =H):
[0102]
[0103] IVe
[0104] Under nitrogen protection, add 2 mmol o-trimethylsilylphenol triflate, 1 mmol N,N'-diisopropylcarbodiimide, and 1 mmol 1-phenyl-2-propyne-1 to a 25 mL reaction tube - Ketone, add 3mL tetrahydrofuran to dissolve. Then add 2.2mmol of anhydrous potassium fluoride and 2.2mmol of 18-crown-6, and react at room temperature (25°C) for 9 hours. The color of the reaction solution changed from colorless to dark red. The reaction solution was concentrated, decolorized and separated on a silica gel column, and a mixed solvent of petroleum ether: ethyl acetate: dichloromethane = 40:1.5:1.5 was used as an eluent to obtain N-{1-[2-(N-isopropyl- N-phenyl)phenyl]-3-benzoyl-2-propynylalkenyl}-isopropylamine 343mg (purity>98%, dark red oily), isolated yield 84%. The NMR data of this compound are as follows: 1 HNMR (400MHz, CD 2 Cl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com