Method for preparing 1,5,9-cyclododecatriene
A technology of cyclododecatriene and butadiene, which is applied in the field of 1,5,9-cyclododecatriene, can solve the problems of adverse effects on CDT yield and not given, and achieve short reaction time, Less polymerization by-products and reduced reaction costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] The reaction was carried out in a four-stage stirred reactor cascade (20 liter volume per reactor). The reaction temperature is kept at 50±1° C. by jacket cooling, and the reactor pressure is adjusted to 0.01-0.12 mpa. The relevant parameters of the four-stage reactor are:
[0092] The pressure relationship of the cascade reaction is: P1>P2>P3>P4
[0093] The catalyst concentration relationship for the cascade reaction is: C1>C2>C3>C4
[0094] The 1,3-butadiene reaction amount relationship of the cascade reaction is: V1>V2>V3>V4
[0095] The temperature relationship of the cascade reaction is T1=T2=T3=T4
[0096] in:
[0097] The pressure of each reactor in the P-cascade reaction
[0098] C-Concentration of catalyst in each reactor in the cascade reaction
[0099] 1,3-butadiene conversion in each reactor in V-cascade reaction
[0100] The temperature of each reactor in the T-cascade reaction
[0101] And in reactor 1 an adjustment was made to obtain a water cont...
Embodiment 2
[0114] Basically the conditions described in Example 1, but adopting diethyl sulfoxide instead of dimethyl sulfoxide in Example 1, and the diethyl sulfoxide was increased to 0.8g / h, and adjusted in reactor 1 A water content of 100 ppm and a sulfur content of 120 ppm were obtained. Under static conditions, add to the reactor:
[0115]
[0116] Other operations are the same as in Example 1 and will not be described in detail.
[0117] Determination of the polymerization product obtained and other by-product types and their content, the results are:
[0118]
[0119] The composition of CDT isomers is:
[0120]
[0121]
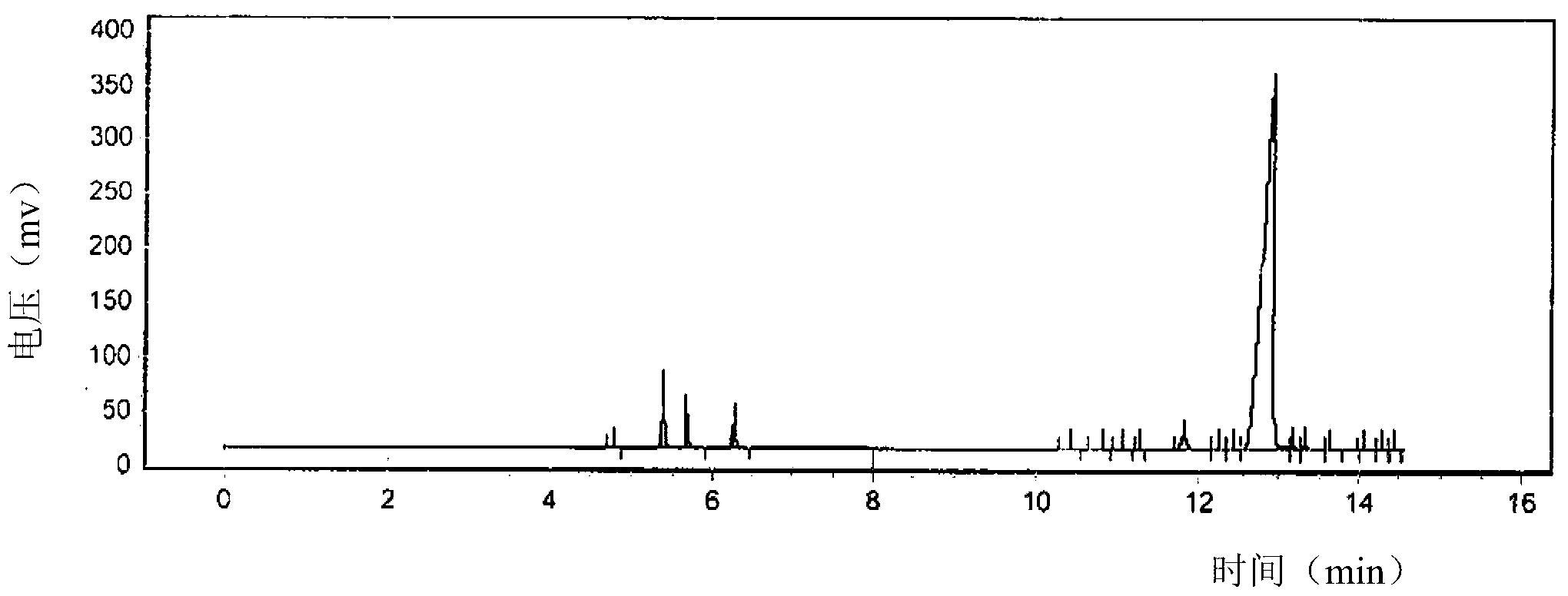
[0122] Before refining, the results of gas chromatographic determination of the reaction product components are shown in Figure 6 . The content results of the components corresponding to the peaks were determined by the area integration method as shown in Table 6 below.
[0123] Table 6
[0124]
Embodiment 3
[0126] Conditions were essentially as described in Example 1, but CDT was used instead of toluene as solvent, dibutylsulfoxide was used instead of dimethylsulfoxide, and adjusted in Reactor 1 to obtain a water content of 120 ppm and a sulfur content of 80 ppm. Add to the reactor under static conditions:
[0127]
[0128] Other operations are the same as in Example 1 and will not be described in detail.
[0129] Determination of the polymerization product obtained and other by-product types and their content, the result:
[0130]
[0131]
[0132] The composition of CDT isomers is:
[0133]
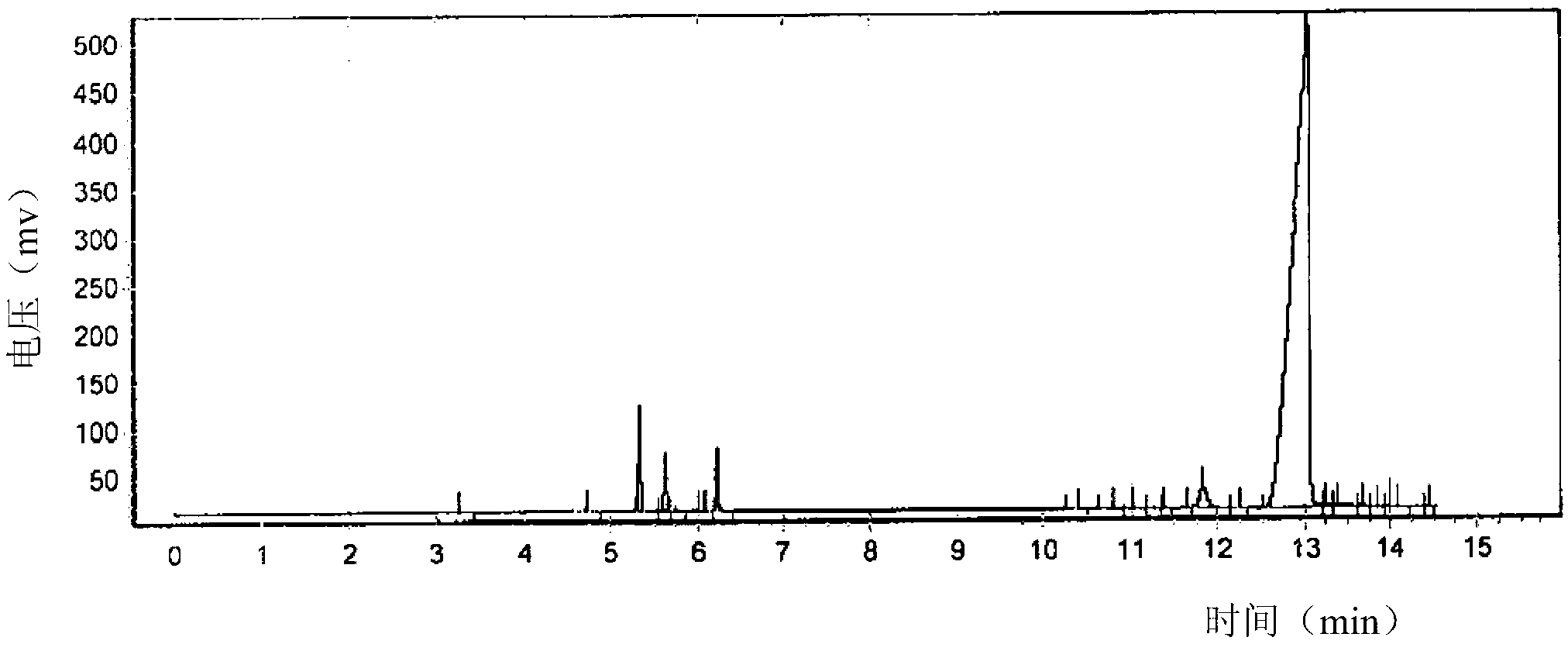
[0134] Before refining, the results of gas chromatographic determination of the reaction product components are shown in Figure 7 . The content results of the corresponding components of each peak determined by the area integration method are shown in Table 7 below.
[0135] Table 7
[0136]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com