Transdermal delivery ointment containing colchicine, and preparation method thereof
A technology for colchicine and ointment, applied in the field of preparation of the ointment, can solve the problems of slow onset of oral dosage forms, discomfort, obvious side effects, etc. fast effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
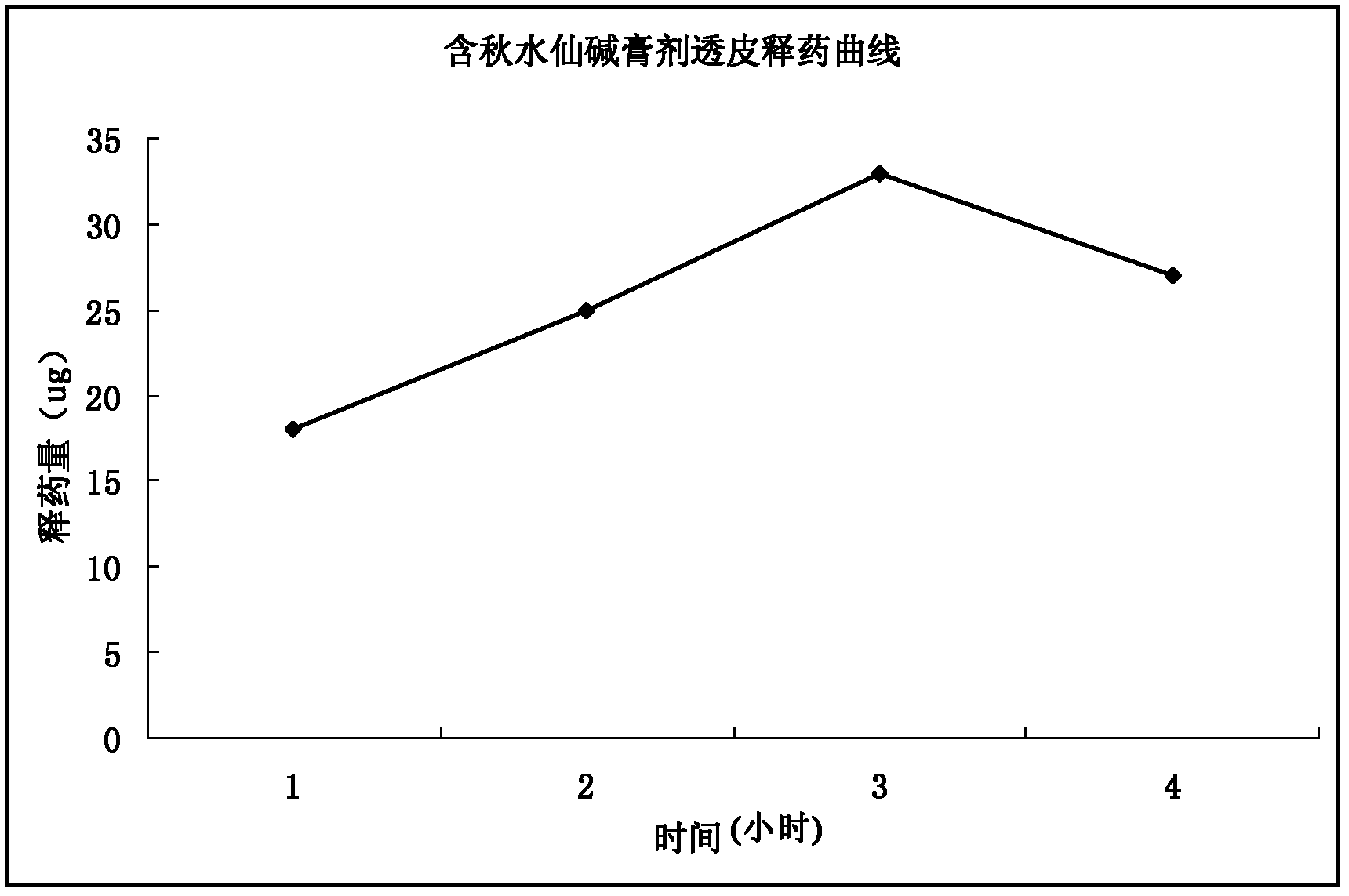
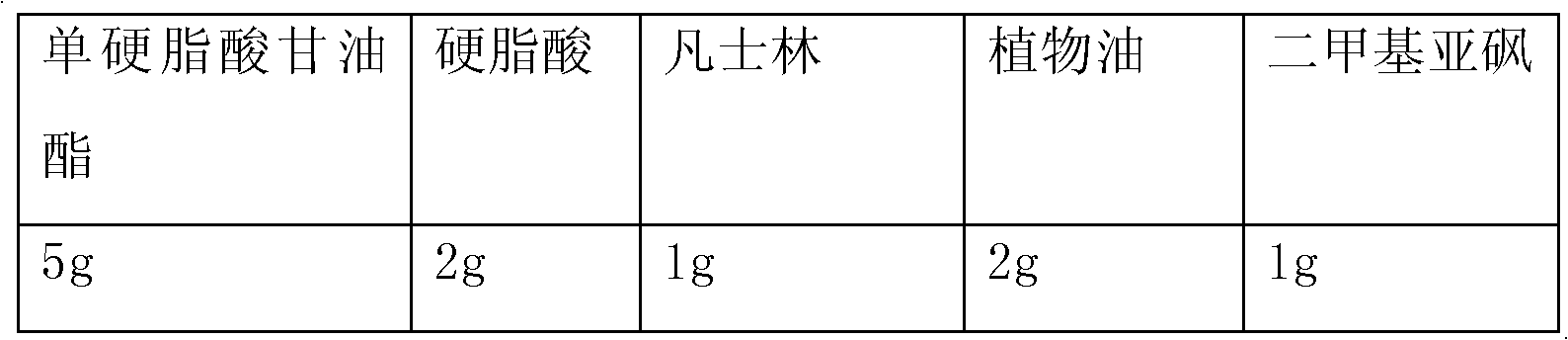
Image
Examples
Embodiment 1
[0027] Water Phase Formulation:
[0028] Colchicine
medical sterile water
Glycerin
25mg
5mL
0.5g
0.3g
[0029] Oil Phase Formula:
[0030] stearic acid
4g
2g
0.75g
0.5g
0.5mL
[0031] Liquid silicone rubber: 1g
[0032] Weigh the water phase, oil phase and liquid silicone rubber respectively according to the formula, fully heat and dissolve the three respectively at 80°C, and then slowly add the water phase to the oil phase under the stirring condition of a rotation speed of 300 rpm to obtain a mixture solution, and then add liquid silicone rubber into the above mixed solution, reduce the stirring speed to 100 rpm, stop heating, and cool to obtain.
Embodiment 2
[0034] Water Phase Formulation:
[0035] Colchicine
[0036] Oil Phase Formula:
[0037] Glyceryl monostearate
[0038] Operation steps: Weigh the water phase and oil phase respectively according to the formula, fully heat and dissolve the two respectively at 60°C, and then slowly add the water phase to the oil phase under stirring at a speed of 400 rpm to obtain a mixed solution , the stirring speed was reduced to 80 rev / min, the heating was stopped, cooled, and it was ready.
Embodiment 3
[0040] Water Phase Formulation:
[0041] Colchicine
medical sterile water
Glycerin
15mg
7mL
1.5g
0.4g
[0042] Oil Phase Formula:
[0043] Glyceryl monostearate
3g
1.5g
0.6g
2g
0.45mL
[0044] Liquid silicone rubber: 2g
[0045] Operation steps: Weigh the water phase, oil phase and liquid silicone rubber according to the formula, fully heat and dissolve the three respectively at 70°C, and then slowly add the water phase to the oil phase under stirring at a speed of 300 rpm , to obtain a mixed solution, then add liquid silicone rubber to the above mixed solution, reduce the stirring speed to 50 rpm, stop heating, and cool to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com