Adsorbing material for removing arsenic from water and preparation method of material
A technology for adsorbing materials and water bodies, applied in the field of arsenic-containing water purification treatment materials and processes, can solve problems such as not being widely used, and achieve the effects of large specific surface area, low cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
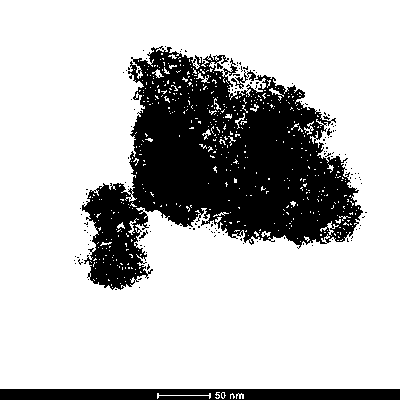
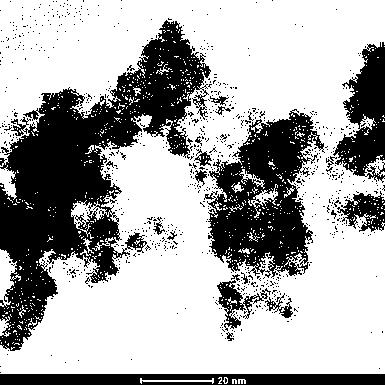
Image
Examples
Embodiment 1
[0031] (1) Weigh 4.040g Fe(NO 3 ) 3 ?9H 2 O, dissolved in 200ml deionized water to obtain Fe 3+ Molar concentration is the iron nitrate solution of 0.05mol / L;
[0032] (2) Weigh 0.02g of surfactant sodium dodecyl sulfonate, add it to the above solution, and stir to make it uniform;
[0033] (3) Add potassium hydroxide to the solution in step (2), and keep stirring until the pH of the solution=10, forming a large amount of insoluble matter;
[0034] (4) Collect a large amount of insoluble matter described in step (3) by suction filtration, wash with deionized water, and suction filtration until the pH of the solution is neutral;
[0035] (5) Collect the solid insolubles in step (4), and dry them in the air at 60°C to obtain amorphous ferric hydroxide powder.
Embodiment 2
[0037] (1) Weigh 40.40g Fe(NO 3 ) 3 ?9H 2 O, dissolved in 200ml deionized water to obtain Fe 3+ Molar concentration is the iron nitrate solution of 0.5mol / L;
[0038] (2) Weigh 0.02g of surfactant sodium dodecyl sulfonate, add it to the above solution, and stir to make it uniform;
[0039] (3) Add ammonia water to the solution in step (2), and keep stirring until the pH of the solution=10, forming a large amount of insoluble matter;
[0040] (4) Collect a large amount of insoluble matter described in step (3) by suction filtration, wash with deionized water, and suction filtration until the pH of the solution is neutral;
[0041] (5) Collect the solid insolubles in step (4), and dry them in the air at 60°C to obtain amorphous ferric hydroxide powder.
[0042] (6) At room temperature, weigh 0.2 g of the above-mentioned adsorption materials in a series of 250 mL Erlenmeyer flasks with stoppers, and add 100 mL of a certain concentration of As ( ) (AsO 2 - ) solution, pla...
Embodiment 3
[0047] (1) Weigh 40.40g Fe(NO 3 ) 3 ?9H 2 O, dissolved in 200ml deionized water to obtain Fe 3+ Molar concentration is the iron nitrate solution of 0.5mol / L;
[0048] (2) Weigh 0.87g Ce(NO 3 ) 3 ?6H 2 O, dissolved in the solution of step (1), yields Ce 3+ Molar concentration is the cerous nitrate solution of 0.01mol / L;
[0049] (3) Weigh 0.02g of surfactant sodium dodecylsulfonate, add it to the above solution, and stir to make it uniform;
[0050] (4) Weigh 0.68 g of hydrogen peroxide with a mass fraction of 30% and pour it into the solution in step (3) (Ce 3+ The molar ratio of hydrogen peroxide to hydrogen peroxide is 1:3), and keep stirring to oxidize trivalent cerous ions into tetravalent ceric ions;
[0051] (5) Add potassium hydroxide to the solution in step (4), and keep stirring until the pH of the solution=10, forming a large amount of insoluble matter;
[0052] (6) Collect a large amount of insoluble matter described in step (5) by suction filtration, was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com