Dearsenification catalyst and preparation method thereof
A catalyst and catalyst quality technology, applied in catalyst activation/preparation, molecular sieve catalysts, chemical instruments and methods, etc., can solve problems such as low arsenic adsorption capacity, adsorption medium pollution, and easy fall-off of activated carbon powder, and achieve simple preparation process and reduced The effect of cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] As introduced in the background technology, there are certain deficiencies in the current de-arsenic catalyst and its preparation method. In addition, in order to solve the above technical problems, in the first typical implementation mode of the present disclosure, a preparation method of the arsenic-removal catalyst is provided , the method includes the following steps:
[0026] Molecular sieves-supported copper salt and nickel salt by impregnation method, dried, and then calcined in air atmosphere to form arsenic removal catalyst precursor-CuO / NiO / MS;
[0027] then in H 2 / N 2 Roasting at 750-850°C for 5-7h in a reducing atmosphere;
[0028] Then oxidize at 750-850°C for 5-7h in an oxidizing atmosphere to form CuNiO 2 / MS metal oxide solid solution, that is, arsenic removal catalyst.
[0029] Through the above method, the copper element and the nickel element can be firstly formed into an alloy, and then oxidized into a metal solid solution, and the synergy of th...
Embodiment 1
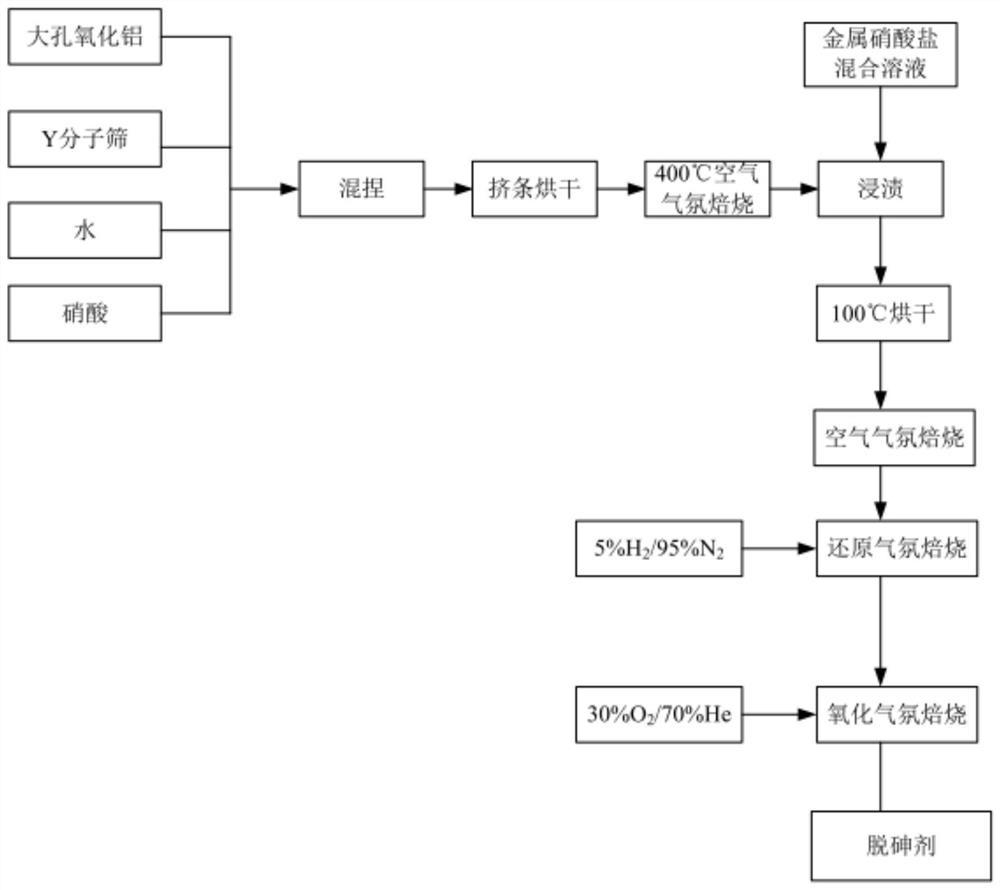
[0061] A preparation method of arsenic removal catalyst, the technological process is as follows figure 1 , the method includes the following steps:
[0062] (1) Preparation of modified macroporous Y-type molecular sieve particles: 100g modified macroporous Y-type molecular sieve powder (specific surface area 700m 2 / g, the pore diameter is about 0.8nm, the content of sodium oxide is 1.5%, the content of rare earth oxide is 12.5%, and the rare earth oxide is La 2 o 3 ), 12g macroporous alumina (specific surface area 200m 2 / g, pore diameter about 1.5nm), 3g nitric acid (78wt%), 40g deionized water are mixed, after kneading, utilize extruder to extrude into the bar of diameter 2.5mm, 100 ℃ of drying 2 hours, crushing length is 3- 8mm cylindrical pellets.
[0063] (2) Loading copper nitrate and nickel nitrate by impregnation method: under normal temperature conditions, dissolve 9.05g copper nitrate trihydrate and 1.95g nickel nitrate hexahydrate in 50g deionized water, and p...
Embodiment 2
[0067] The modified macroporous Y-type molecular sieve particles were prepared by the same method as in Embodiment 1.
[0068] Load copper nitrate and nickel nitrate by impregnation method: under normal temperature conditions, dissolve 15g copper nitrate trihydrate and 3.25g nickel nitrate hexahydrate in 50g deionized water, put 100g macroporous Y-type molecular sieve particles into the water, and pass the equal volume impregnation method , load copper nitrate and nickel nitrate on a large-pore Y-type molecular sieve, and dry at 100°C.
[0069] Aftertreatment process and with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com