A kind of arsenic removal catalyst and preparation method thereof
A catalyst and catalyst quality technology, applied in catalyst activation/preparation, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems affecting the ability of arsenic to form complexes, low arsenic adsorption capacity, and pollution of adsorption media, etc. Excellent arsenic removal ability, high arsenic adsorption capacity, simple preparation process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] As introduced in the background technology, there are certain deficiencies in the current de-arsenic catalyst and its preparation method. In addition, in order to solve the above technical problems, in the first typical implementation mode of the present disclosure, a preparation method of the arsenic-removal catalyst is provided , the method includes the following steps:
[0026] Molecular sieves-supported copper salt and nickel salt by impregnation method, dried, and then calcined in air atmosphere to form arsenic removal catalyst precursor-CuO / NiO / MS;
[0027] then in H 2 / N 2 Roast at 750-850°C for 5-7h in a reducing atmosphere;
[0028] Then oxidize at 750-850°C for 5-7h in an oxidizing atmosphere to form CuNiO 2 / MS metal oxide solid solution, that is, arsenic removal catalyst.
[0029] Through the above method, the copper element and the nickel element can be firstly formed into an alloy, and then oxidized into a metal solid solution, and the synergy of the t...
Embodiment 1
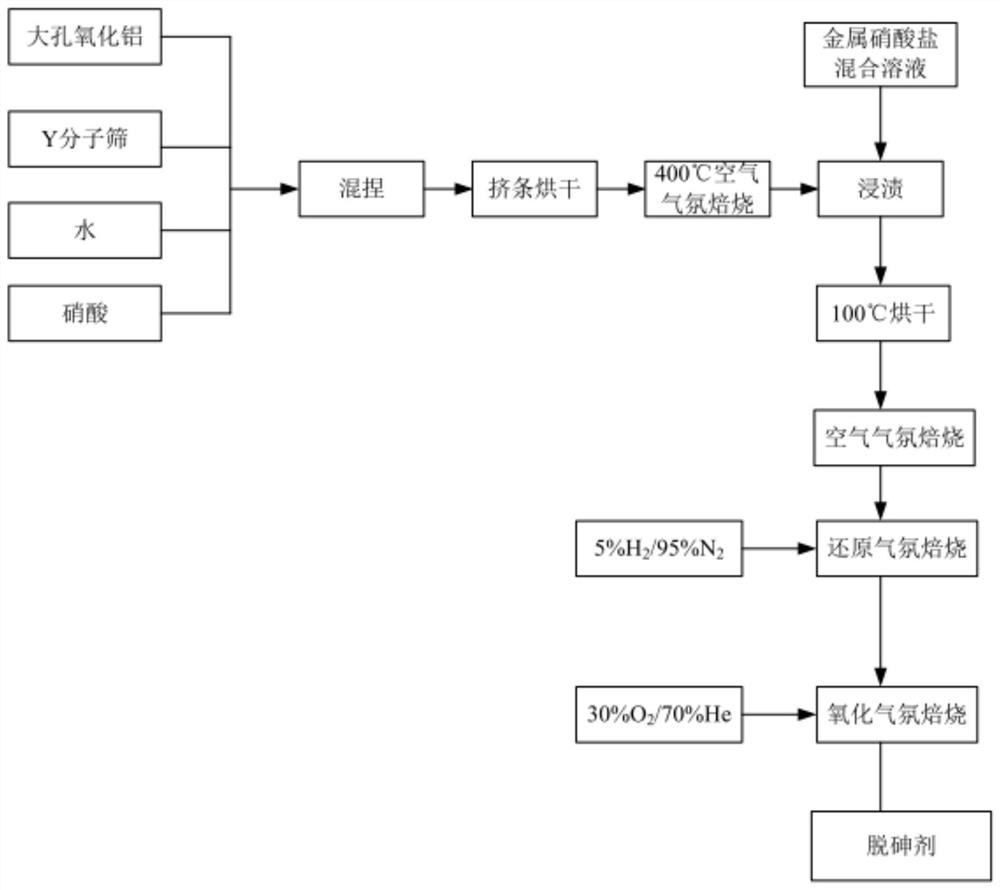
[0061] A preparation method of arsenic removal catalyst, the technological process is as follows figure 1 , the method includes the following steps:
[0062] (1) Preparation of modified macroporous Y-type molecular sieve particles: 100g modified macroporous Y-type molecular sieve powder (specific surface area 700m 2 / g, the pore diameter is about 0.8nm, the content of sodium oxide is 1.5%, the content of rare earth oxide is 12.5%, and the rare earth oxide is La 2 o 3 ), 12g macroporous alumina (specific surface area 200m 2 / g, pore diameter about 1.5nm), 3g nitric acid (78wt%), 40g deionized water are mixed, after kneading, utilize extruder to extrude into the bar of diameter 2.5mm, 100 ℃ of drying 2 hours, crushing length is 3- 8mm cylindrical pellets.
[0063] (2) Loading copper nitrate and nickel nitrate by impregnation method: under normal temperature conditions, dissolve 9.05g copper nitrate trihydrate and 1.95g nickel nitrate hexahydrate in 50g deionized water, and p...
Embodiment 2
[0067] The modified macroporous Y-type molecular sieve particles were prepared by the same method as in Embodiment 1.
[0068] Load copper nitrate and nickel nitrate by impregnation method: under normal temperature conditions, dissolve 15g copper nitrate trihydrate and 3.25g nickel nitrate hexahydrate in 50g deionized water, put 100g macroporous Y-type molecular sieve particles into the water, and pass the equal volume impregnation method , load copper nitrate and nickel nitrate on a large-pore Y-type molecular sieve, and dry at 100°C.
[0069] Aftertreatment process and with embodiment 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com