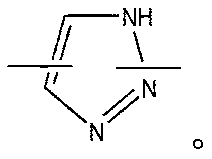
Radial shape of polymer compound containing iodine, preparation method thereof, and ct contrast medium composition containing same
A small molecular compound, radial technology, applied in the preparation of X-ray contrast agents, medical preparations containing active ingredients, radioactive carriers, etc., can solve problems such as poor image quality, prolong the duration of contrast enhancement, high toxicity, etc., and achieve low Effects of polydispersity, high reproducibility, and simplicity of preparation and purification methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0126] In the preparation method of the iodine-containing radial macromolecules of the present invention, examples of circular or spherically symmetrical small molecule compounds, radial macromolecules, surface functional groups, iodine-containing compounds, peptides, and biocompatible polymers are the same as those described above. same.
[0127] In the preparation method of the embodiment of the present invention, biocompatible polymers are used to derivatize about 15-50% of the functional groups on the surface of the core, and then the excess iodine-containing compound is treated to react with the remaining functional groups of the core. Therefore, the iodine content in radial macromolecules can be controlled in the range of about 15-50%.
[0128] In addition, the present invention also provides a CT contrast medium composition containing iodine-containing radial macromolecule as an active ingredient.
[0129] In the present invention, the preferred iodine content of the i...
preparation example 1
[0131] [Preparation Example 1] Preparation of Cyclized Neutral Phthalimide Derivatives (8)
[0132]
[0133]Triethylamine (0.50 mL, 3.59 mmol) was added to compound 6 (4,5,6,7-tetraiodobenzofuran-1,3-dione (TIPN), 2.40 g, 3.68 mmol) and compound 7 ( N-tert-butoxycarbonyl-ethylenediamine (N-tert-Boc-ethylenediamine), 200mg, 1.22mmol) in dimethylsulfoxide (DMSO) (5mL) solution. The reaction was heated at 40 °C and stirred under dry argon for 16 hours. Pass the reaction mixture through a size exclusion chromatography (SEC, Size-Exclusion Chromatography) column (model: Bio-Beads S-X1, H36cm×O.D.4.5cm) in N,N-dimethylformamide (DMF), manufacturer: Bio-Rad) to remove DMSO, and concentrated under reduced pressure, the crude product was purified on silica gel (10:1 dichloromethane (CH 2 Cl 2 ) / acetone) to obtain 60.1 mg of compound 8 (75.7 μmol, 6%) as a yellow powder.
[0134] R f : 0.63[Silicone, 10:1CH 2 Cl 2 / acetone];
[0135] 1 H NMR (600MHz, DMSO-d 6 )δ6.89(t,1H,J=...
preparation example 2
[0138] [Preparation Example 2] Preparation of Cyclized Neutral Phthalimide Derivatives (10)
[0139]
[0140] Triethylamine (0.82 mL, 5.88 mmol) was added to a solution of compound 6 (TIPN, 3.84 g, 5.89 mmol) and compound 9 (ethanolamine, 300 mg, 4.91 mmol) in DMSO (8 mL). The reaction was heated at 50 °C and stirred under dry argon for 19 hours. The reaction mixture was passed through a SEC column (model: Bio-Beads S-X1, H36cm×O.D.4.5cm, manufacturer: Bio-Rad Company) in DMF to remove DMSO, concentrated under reduced pressure, and the crude product was separated on silica gel ( 10:1CH 2 Cl 2 / acetone) for chromatographic separation to obtain 1.11 g of compound 10 (1.6 mmol, 33%) as a yellow powder.
[0141] R f : 0.37[Silicone, 10:1CH 2 Cl 2 / acetone];
[0142] 1 H NMR (600MHz, DMSO-d 6 )δ3,62(t,2H,J=5,8Hz,H 1 ), 3.55(q,2H,J=5.8Hz,H 2 );
[0143] 13 C NMR (150MHz, DMSO-d 6 ) δ 164.2, 135.5, 134.4, 104.0, 57.5, 41.3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com