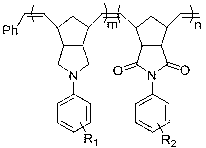
Norbornene monomer, as well as polymer and preparation method thereof
A norbornene and polymer technology, which is applied in the fields of norbornene and its polymers and preparation, can solve the problem of large distance between donor side chain units and acceptor side chain units, weakened π-π interaction, side chain Problems such as the reduction of arrangement regularity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
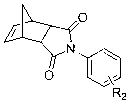
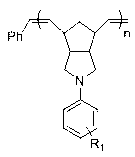
[0055] Example 1: Preparation of N-(4-tert-butyldimethylsilyloxyphenyl) norbornene pyrrolidine (M1)
[0056] The synthetic route is as follows:
[0057]
[0058] Specific steps:
[0059] (1) Dissolve 150 ml of norbornene dioic anhydride (8.2 g, 50 mmol) and 4-aminophenol (5.46 g, 50 mmol) in glacial acetic acid, and react at room temperature for 15 h. The reaction solution was dropped into 500 ml of ice water, and a large amount of white precipitates were precipitated immediately. After filtration, vacuum drying was performed for 48 h to obtain 11 g of white powder, yield: 86%.
[0060] (2) Under a nitrogen atmosphere, the product N-(4-hydroxyphenyl) norbornene imide (6.4 g, 25 mmol) obtained in step (1) was dissolved in 120 ml of dry CH 2 Cl 2 , TBS-Cl (7.5 g, 50 mmol) and imidazole (3.4 g, 50 mmol) were added under ice cooling. A large amount of salt was formed immediately, and it was naturally raised to room temperature, and reacted for 12 h. Remove the imidazolium ...
Embodiment 2
[0062] Example 2: Preparation of N-(3,5-difluorophenyl) norbornene imide (M2)
[0063] The synthetic route is as follows:
[0064]
[0065] Specific steps: under nitrogen atmosphere, dissolve norbornene dianhydride (9.84 g, 60 mmol) and 3,5-difluoroaniline (6.45 g, 50 mmol) in 50 mL of anhydrous 1,2-dichloroethane , 0.3 equivalents of DMAP (1.83 g, 15 mmol) was added to the reaction solution. Reflux at 90 °C for 36 h, the reaction was complete. After repeated washing, extraction and recrystallization, 11.2 g of white crystals were obtained with a yield of 81.5%. The resulting product is N-(3,5-difluorophenyl)norbornenimide (M2). 1 H NMR (500 MHz, CDCl 3 , δ): 6.83 (m, 1H, CFC H CF), 6.81-6.77 (m, 2H, NCC H C H ), 6.25 (s, 2H, C H =C H ), 3.51 (s, 2H, CHC H CO), 3.43 (s, 2H, C H CH 2 C H ), 1.80-1.60 (m, 2H, CHC H 2 CH); 13 C NMR (500 MHz, CDCl 3 , δ): 175.93 (CH C =O), 163.66 (CH C F), 161.79 (N C CH), 134.66 (CH= C HCH), 110.04 (NC C H), 104.1 (FC C ...
Embodiment 3
[0066] Example 3: Preparation of electron donor type norbornene homopolymer
[0067] In a 25 mL Schlenk reaction tube, add electron-donor norbornene-based monomer N-(4-tert-butyldimethylsilyloxyphenyl)norbornene pyrrolidine (formula (M1), 341 mg, 1 mmol) with 3 mL CH 2 Cl 2 , Grubbs third generation catalyst (H 2 IMes)(3-BrPy) 2 (Cl) 2 Ru(CHPh) (16.5 mg, 0.02 mmol) with 2 mL CH 2 Cl 2 , the molar ratio of monomer to catalyst is 50:1. The two reaction tubes were frozen with liquid nitrogen, evacuated, and replaced with nitrogen three times. After thawing and rising to room temperature, the monomer reaction tube was placed in an oil bath at 30 °C for 5 min. Under a nitrogen atmosphere, the catalyst solution was added into the monomer reaction tube with a syringe, and the system was placed in an oil bath at 30 °C for 1 h. Stop the reaction, add 2 drops of vinyl ether to the system, and stir at room temperature for 30 min. The reaction solution was dropped into methanol ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com