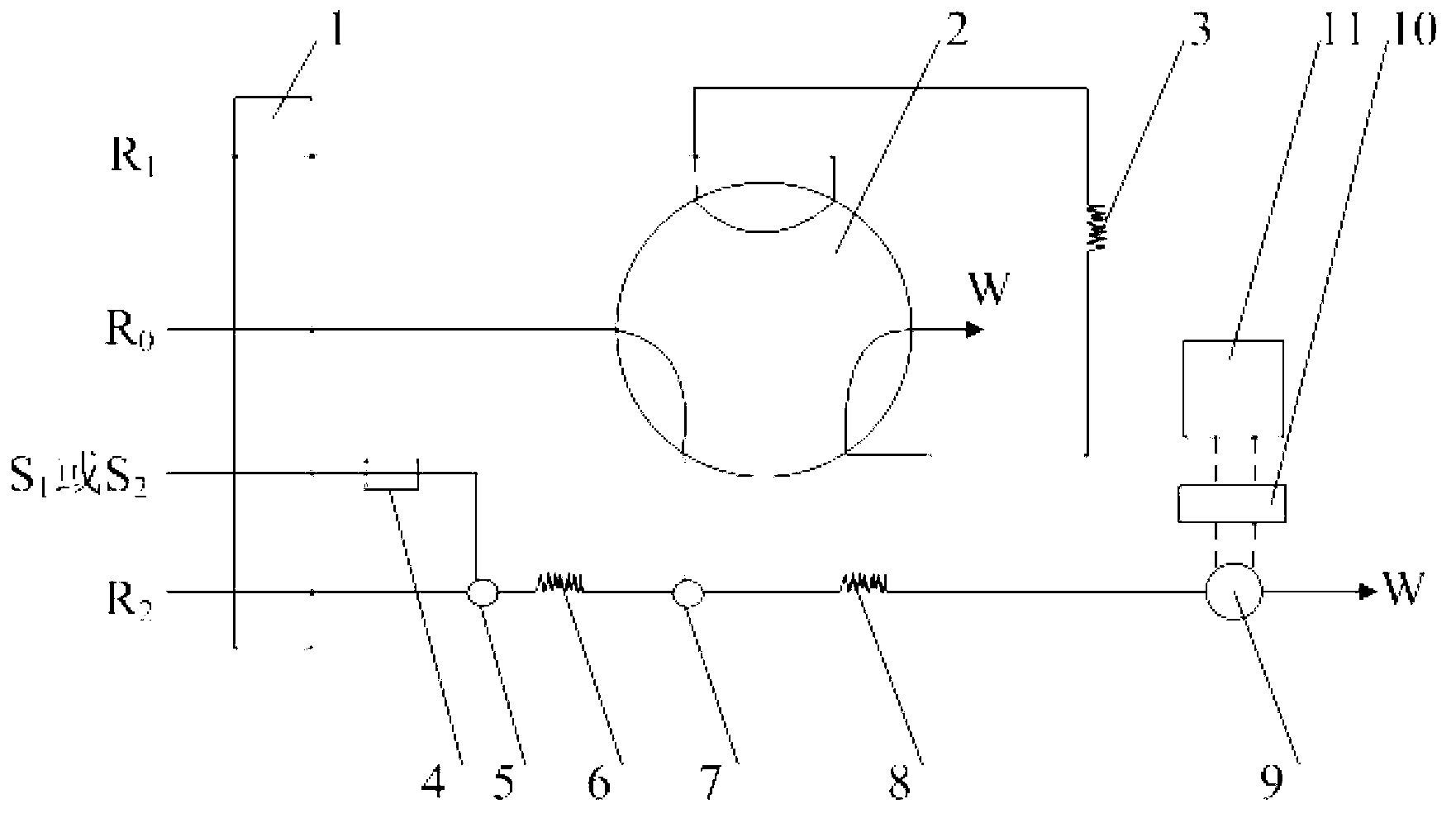
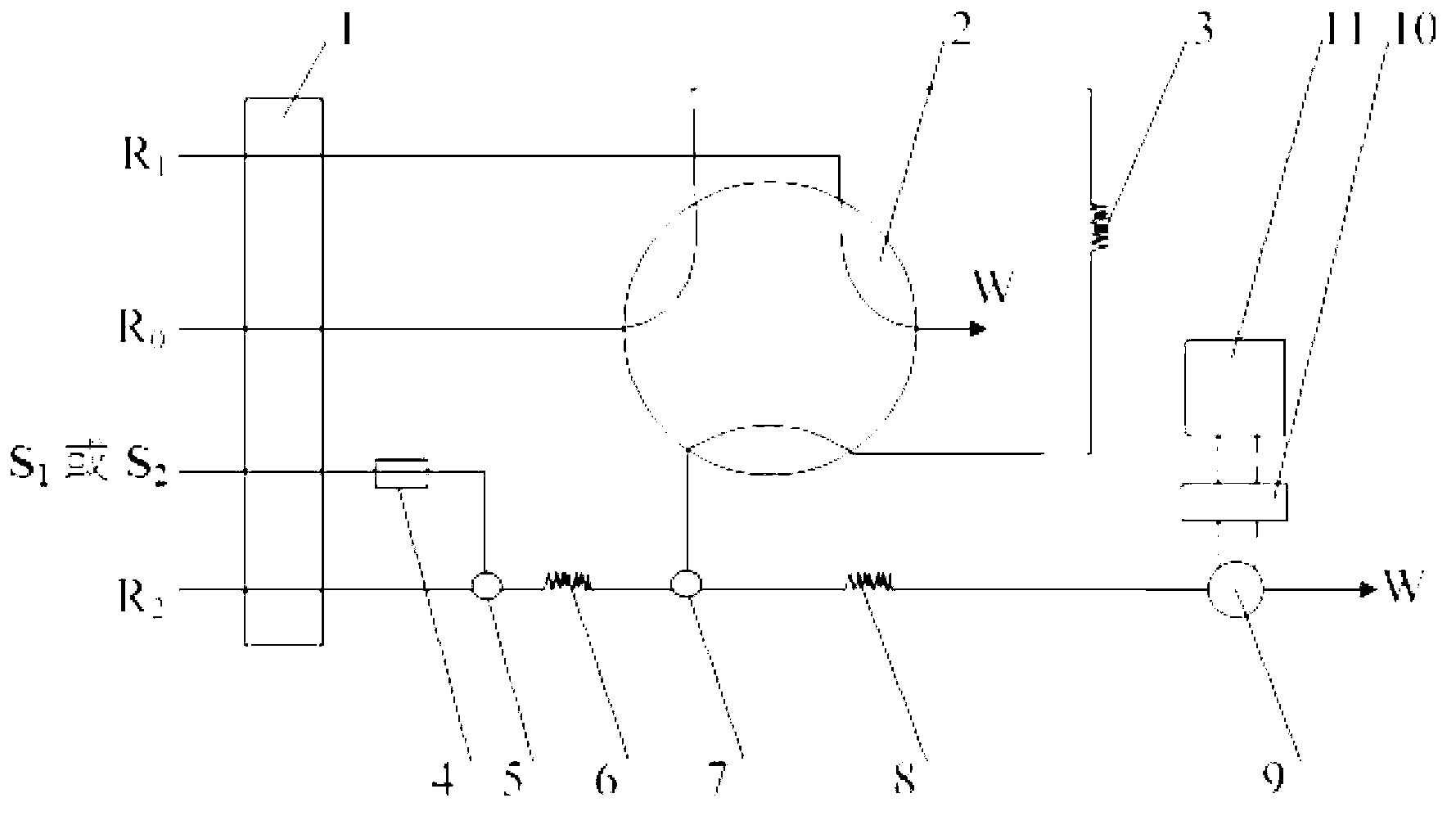
Automatic analysis method of formaldehyde in water sample
An automatic analysis and formaldehyde technology, applied in the direction of analysis materials, instruments, etc., can solve the problems of poor anti-interference ability, slow analysis speed, and complicated operation, and achieve the effects of high precision, reduced analysis cost, and improved analysis speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This embodiment prepares the Cl containing quaternary amine group - Type exchange resin, the preparation method is as follows:
[0038] 1. Take styrene and divinylbenzene as raw materials, take benzoyl peroxide as catalyst, the quality of styrene: the quality of divinylbenzene: the quality of benzoyl peroxide=600:60:1; Put vinylbenzene and benzoyl peroxide into the reaction container, carry out polymerization reaction at normal pressure and 90°C to generate styrene-divinylbenzene spherical copolymer beads, and the reaction time is 15h;
[0039] ② Sorting the styrene-divinylbenzene spherical copolymer beads prepared in step ① with a standard sieve to obtain styrene-divinylbenzene spherical copolymer beads with a particle size of 30 μm to 50 μm;
[0040] ③Put styrene-divinylbenzene spherical copolymer beads with a particle size of 30 μm to 50 μm in a reaction vessel, add chloromethyl ether, dichloromethane and nitromethane and stir evenly, then at room temperature and no...
Embodiment 2
[0043] In this embodiment, the standard sample is tested to investigate the precision of the method of the present invention. The steps are as follows:
[0044] 1. Preparation of standard samples
[0045] (1) Prepare a standard stock solution of formaldehyde with a concentration of 1000 mg / L: Take 2.8 mL of formaldehyde solution with a content of 36% to 38%, put it into a 1L volumetric flask, add deionized water to dilute to the mark, and shake well. Calibrated by iodometric method. The prepared formaldehyde standard stock solution can be stored at 4°C for half a year.
[0046] (2) Prepare 0.200 mg / L formaldehyde standard sample: pipette 0.02 mL of the formaldehyde standard stock solution prepared in step (1) into a 100 mL volumetric flask, and dilute to the mark line with deionized water.
[0047] 2. Chromogenic solution R 1 preparation
[0048] Weigh 0.3 g ferric ammonium sulfate (NH 4 Fe(SO 4 ) 2 12H 2 O), dissolved in hydrochloric acid with a concentration of 0.03...
Embodiment 3
[0059] In the present embodiment, the tested samples are three kinds of tanning wastewater, which are respectively 1# sample, 2# sample and 3# sample, and its analysis steps are as follows:
[0060] 1. Preparation of standard samples
[0061] ① Prepare a standard stock solution of formaldehyde with a concentration of 1000 mg / L
[0062] The method of preparing formaldehyde standard stock solution is identical with embodiment 2.
[0063] ② Prepare a series of standard samples
[0064] Dilute the formaldehyde standard stock solution prepared in step ① with deionized water to prepare a series of standard samples. The concentrations of formaldehyde in each standard sample are: 0, 0.005 mg / L, 0.010 mg / L, 0.050 mg / L, 0.100 mg / L, 0.300 mg / L, 0.500 mg / L, 0.700 mg / L, 0.900 mg / L and 1.00 mg / L.
[0065] 2. Chromogenic solution R 1 , reference solution R 0 and reaction solution R 2 Same as Example 2, and the preparation method is the same as Example 2.
[0066] The chemical reagent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com