Method for synthesizing hydroxyapatite from alkali residue
A hydroxyapatite and alkali slag technology, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., to achieve the effect of reducing synthesis cost, high product purity, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
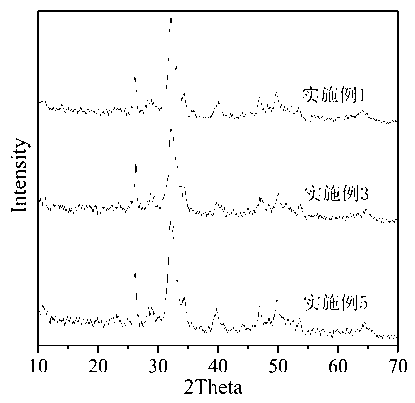
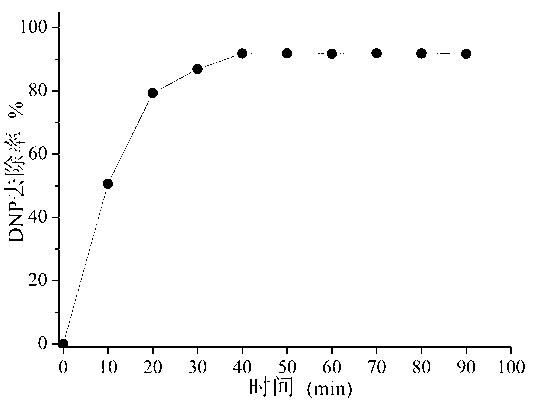
Image
Examples
Embodiment 1
[0031] Weigh 50 g of alkali slag powder that has been dried and ground to 140 mesh, add it to 1 mol / L hydrochloric acid at a solid-to-liquid ratio of 1 g:10 mL, stir the reaction fully, and filter to obtain the filtrate. The pH of the filtrate was adjusted to 9.5 with aqueous ammonia, and the precipitate was removed by filtration. The concentration of Ca ions in the supernatant (calcium source) was quantified by inductively coupled plasma atomic emission spectrometry, and the result was 20 g / L. Prepare 0.3mol / L diammonium hydrogen phosphate solution. Take 100mL of calcium source and slowly add it into 100mL of 0.3mol / L diammonium hydrogen phosphate solution, fully stir and react for 2 hours, then age for 12 hours. The white solid was separated by filtration, washed with water and ethanol, and then dried to obtain a white powder, which was hydroxyapatite.
Embodiment 2
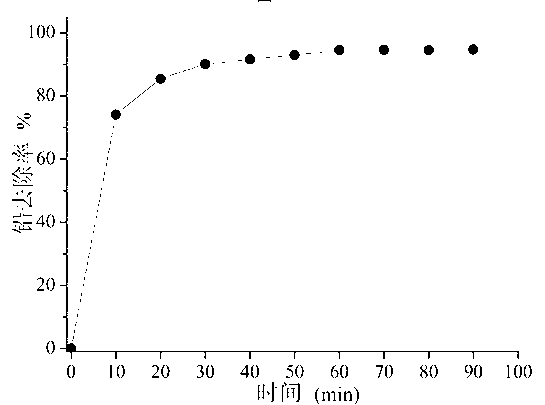
[0033] Take 0.1 g of the hydroxyapatite prepared in Example 1 and add it to 100 mL of wastewater with a lead concentration of 50 mg / L. The change of its removal rate with time is shown in figure 2 , it can be seen from the figure that the removal rate of lead is fast, and the removal rate of 90% can be reached in 30 minutes, and the balance can be reached after 1 hour, and the equilibrium removal rate is 94.2%.
Embodiment 3
[0035] Weigh 70g of alkali slag powder that has been dried and ground to 80 mesh, add it to 1.5mol / L hydrochloric acid at a solid-to-liquid ratio of 1 g:20 mL, stir the reaction fully, and filter to obtain the filtrate. The pH of the filtrate was adjusted to 10.5 with aqueous ammonia, and the precipitate was removed by filtration. The concentration of Ca ions in the supernatant (calcium source) was quantified by inductively coupled plasma atomic emission spectrometry, and the result was 25.5 g / L. Prepare 0.38mol / L sodium dihydrogen phosphate solution. Take 100mL of calcium source and slowly add it into 100mL of 0.38mol / L sodium dihydrogen phosphate solution, fully stir and react for 1 hour, then age for 24 hours. The white solid was separated by filtration, washed with water and methanol for several times, and then dried to obtain a white powder, namely hydroxyapatite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com