Preparation method of high-purity strontium ranelate
A strontium ranelate and high-purity technology, which is applied in the field of preparation of high-purity strontium ranelate, can solve problems such as environmental risks, high environmental pressure, and uneven reaction, so as to improve reaction yield and product quality, and reduce waste water consumption. The effect of producing, stable mass yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The preparation of embodiment 1 intermediate I
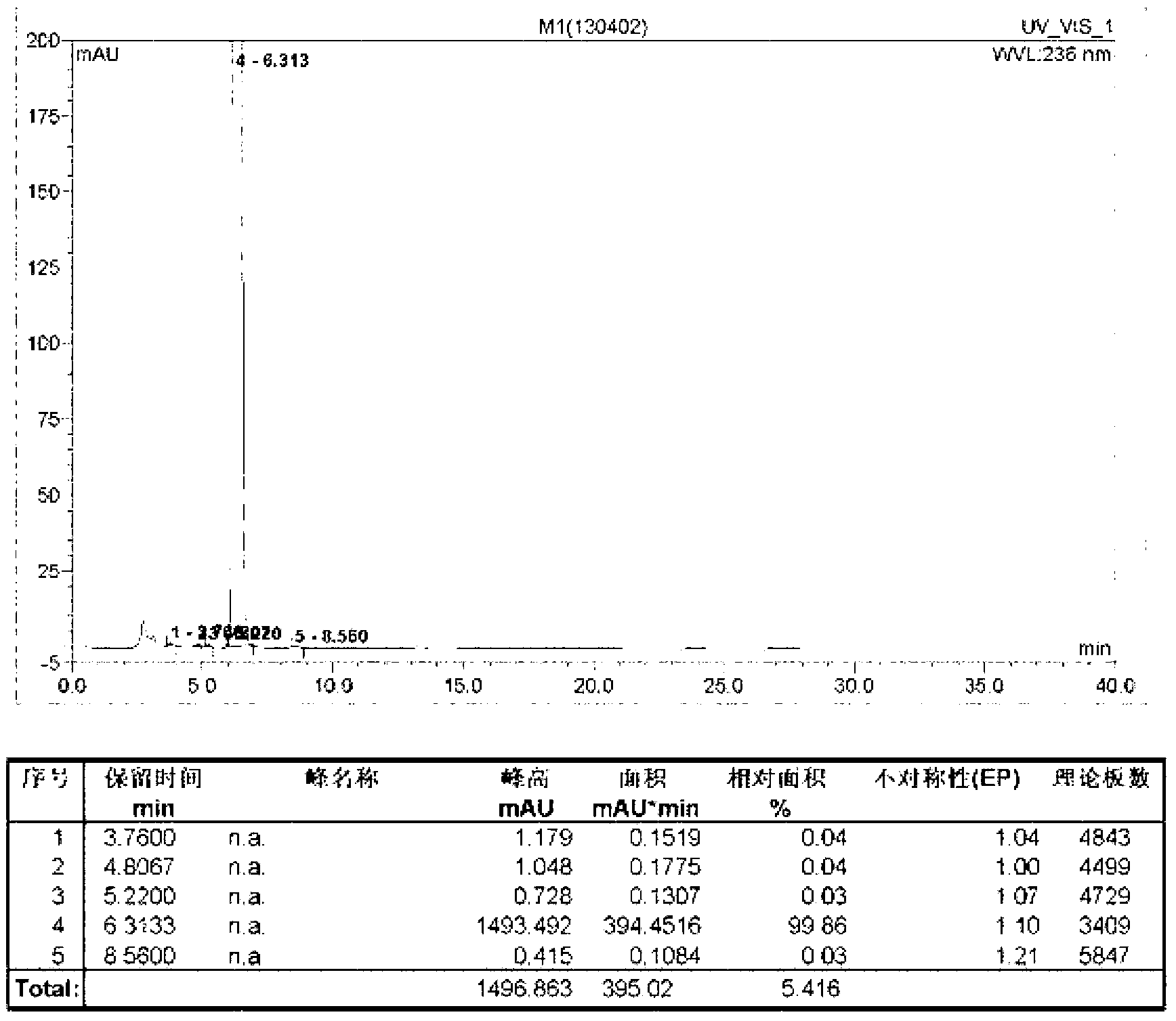
[0044] Add 20.0kg of diethyl acetone dicarboxylate, 6.6kg of malononitrile, 3.2kg of sulfur, 40.0kg of ethanol into a 100L enamel reaction kettle, and then add 0.16kg of ammonium sulfide. Ethylamine, after the feeding is completed, the temperature of the materials in the reactor is raised to reflux for 1.0h of reaction. After the reaction is completed (use TLC to detect whether the reaction has reached the end point, the developer: ethyl acetate and chloroform in a volume ratio of 1:4 mixed), lowered to room temperature, and grown in an ice-water bath for 1.0 h. The filter cake was collected by filtration, refined by adding 95% ethanol by volume, filtered, and dried to obtain 22.9 kg of intermediate I, with a purity of 99.86% and a yield of 82.1%.
[0045] The HPLC figure of intermediate I that the present embodiment makes is as follows figure 1 shown.
Embodiment 2
[0046] Embodiment 2 Preparation of tetraethyl ranelate
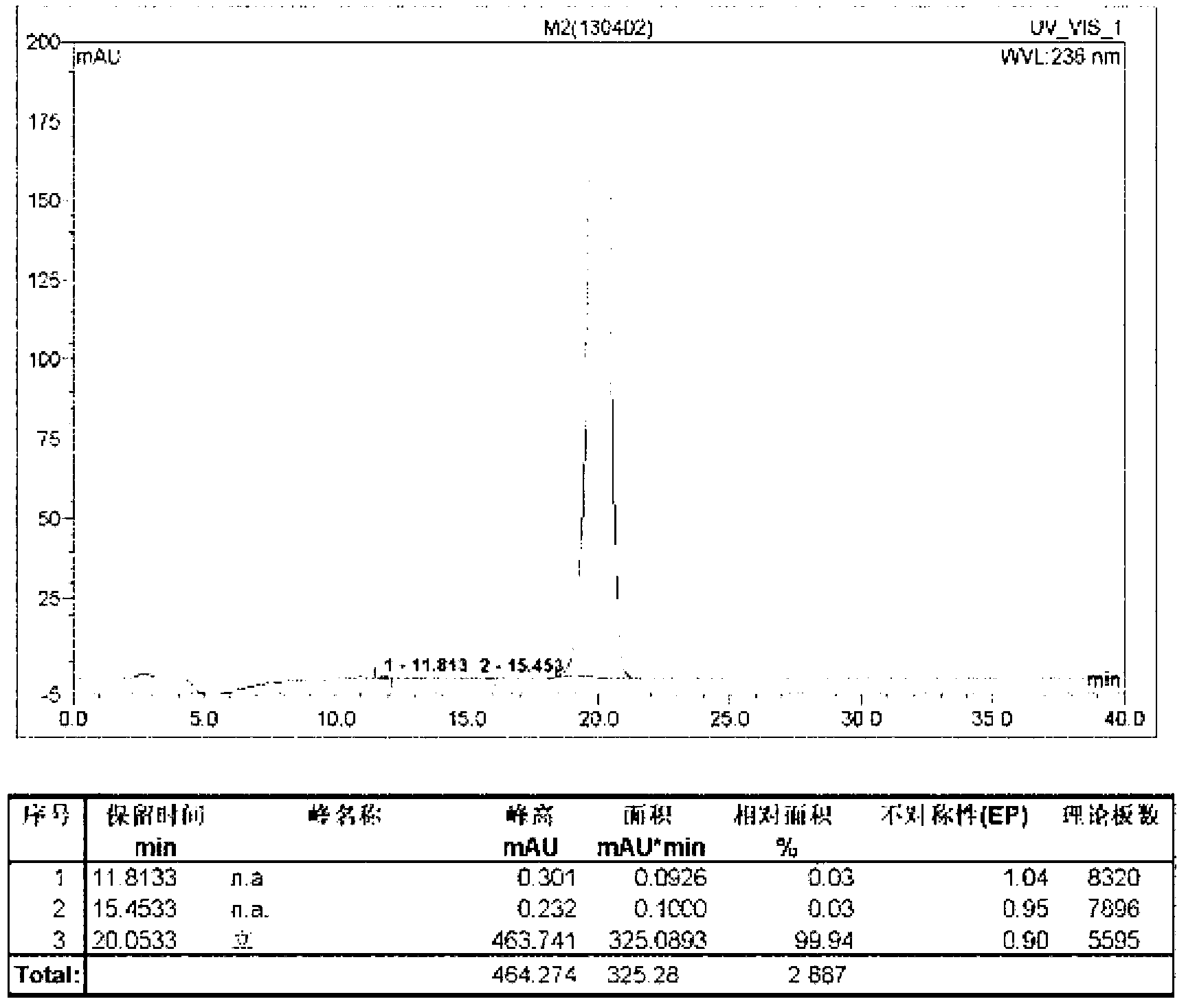
[0047] Add 22.9kg intermediate I, 30.0kg acetone, 28.8kg ethyl bromoacetate, 19.8kg anhydrous potassium carbonate and 0.5kg potassium iodide fine powder prepared by the method in Example 1 successively in the 100L enamel reaction kettle, after stirring at room temperature , heat up, keep the temperature at 60°C and reflux for 1.5h, the color of the reaction solution gradually changes from golden yellow to red. After the reaction is completed (TLC is used to detect whether the reaction has reached the end point, the developer: ethyl acetate and chloroform are mixed at a volume ratio of 1:4), cooled to room temperature, filtered to remove potassium carbonate, and the filtrate is concentrated under reduced pressure at 45°C To dryness, a brown oil was obtained, which was stirred and dissolved by adding 180.0kg of 95% by volume ethanol. After hot filtration, the temperature was naturally cooled and crystallized, and the cryst...
Embodiment 3
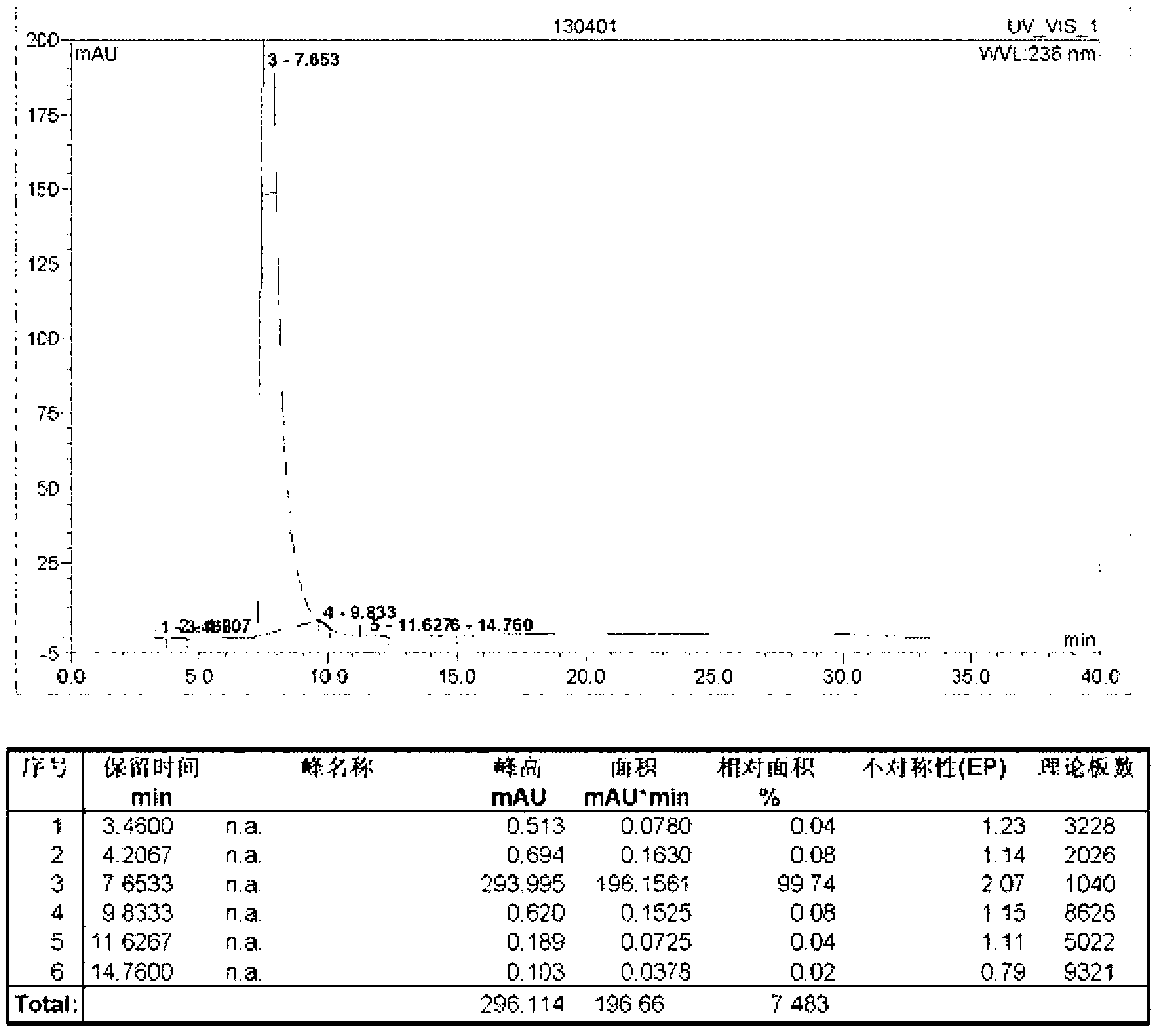
[0050] Preparation of Intermediate I
[0051] Add 20.0kg of diethyl acetone dicarboxylate, 6.6kg of malononitrile, 3.2kg of sulfur, 40.0kg of ethanol into a 100L enamel reaction kettle, and then add 0.096kg of ammonium sulfide. Ethylamine, after the feeding is completed, the temperature of the materials in the reactor is raised to reflux for 1.0h of reaction. After the reaction is completed (use TLC to detect whether the reaction has reached the end point, the developer: ethyl acetate and chloroform in a volume ratio of 1:4 mixed), lowered to room temperature, and grown in an ice-water bath for 1.0 h. The filter cake was collected by filtration, refined by adding 95% ethanol by volume, filtered, and dried to obtain 22.9 kg of intermediate I, with a purity of 99.69% and a yield of 81.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com