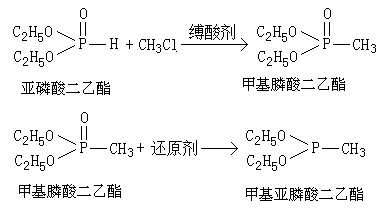
Synthetic method of diethyl methyl-phosphonite and glufosinate-ammonium
A technology of diethyl methyl phosphonite and diethyl methyl phosphonate, applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, organic chemistry, etc., can solve the intermediate methyl Phosphonyl dichloride is extremely unstable, etc., and achieves the effects of high atomic utilization, simple reaction process, and wide application value.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In a 1L autoclave, add 138g of diethyl phosphite and 101g of anhydrous triethylamine, close the autoclave, and use N 2 The air in the kettle was replaced, the temperature was raised to 110°C with stirring, and methyl chloride gas was slowly introduced to maintain the pressure in the kettle at 0.4-0.8 MPa during the reaction. After 4 hours of reaction, the temperature was lowered and the pressure was released, triethylamine hydrochloride was removed by suction filtration, the filtrate was transferred to a 1000 mL four-necked flask, the temperature was cooled to 0 °C with an ice-salt bath, and 200 mL of a THF solution dissolved in 19 g of lithium tetrahydrogen was slowly added dropwise. After the dropwise addition, continue to insulate and stir for 120 min. The temperature was slowly raised for distillation, and 126.1 g of fractions with a boiling point of 120-122°C were collected. The content of diethyl methylphosphonite was detected to be 98.5%, and the yield was 91.3%....
Embodiment 2
[0037] In a 1L autoclave, add 138g of diethyl phosphite and 222g of tri-n-butylamine, close the autoclave, and use N 2 Replace the air in the kettle, stir and raise the temperature to 100°C, and slowly feed in methyl chloride gas to maintain the pressure in the kettle at 0.4-0.8MPa. After reacting for 3 hours, the temperature was lowered and the pressure was released, tributylamine hydrochloride was removed by suction filtration, the filtrate was transferred to a 1000 mL four-neck flask, the temperature was cooled to 5 °C with an ice-salt bath, and 200 mL of a methyl tetrahydrofuran solution dissolved in 19 g of lithium tetrahydrogen was slowly added dropwise. After the dropwise addition, continue to insulate and stir for 90 min. The temperature was slowly raised for distillation, and 120.8 g of fractions with a boiling point of 120-122°C were collected. The content of diethyl methylphosphonite was detected to be 98.8%, and the yield was 87.8%.
Embodiment 3
[0039] In a 1L autoclave, add 138g of diethyl phosphite and 367.5g of triphenylamine, close the autoclave, and use N 2 Replace the air in the kettle, stir and raise the temperature to 120°C, slowly feed in methyl chloride gas to maintain the pressure in the kettle at 0.4-0.8MPa. After reacting for 2 hours, the temperature was lowered and the pressure was released, triphenylamine hydrochloride was removed by suction filtration, the filtrate was transferred to a 1000 mL four-neck flask, the temperature was lowered to 10°C with a cold water bath, and 200 mL of ether solution dissolved in 24 g of sodium hydride was slowly added dropwise. After the dropwise addition, continue to insulate and stir for 60 min. The temperature was slowly raised to distill, and 119.5 g of fractions with a boiling point of 120-122°C were collected. The content of diethyl methylphosphonite was detected to be 98.9%, and the yield was 86.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com