H1N1/H5N1 type avian influenza virus detection kit and application thereof
A detection kit, avian influenza virus technology, applied in the direction of DNA/RNA fragmentation, microbial determination/inspection, recombinant DNA technology, etc. , easy cross-contamination and other problems, to achieve the effect of shortening the detection time, easy operation, and avoiding mutual interference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
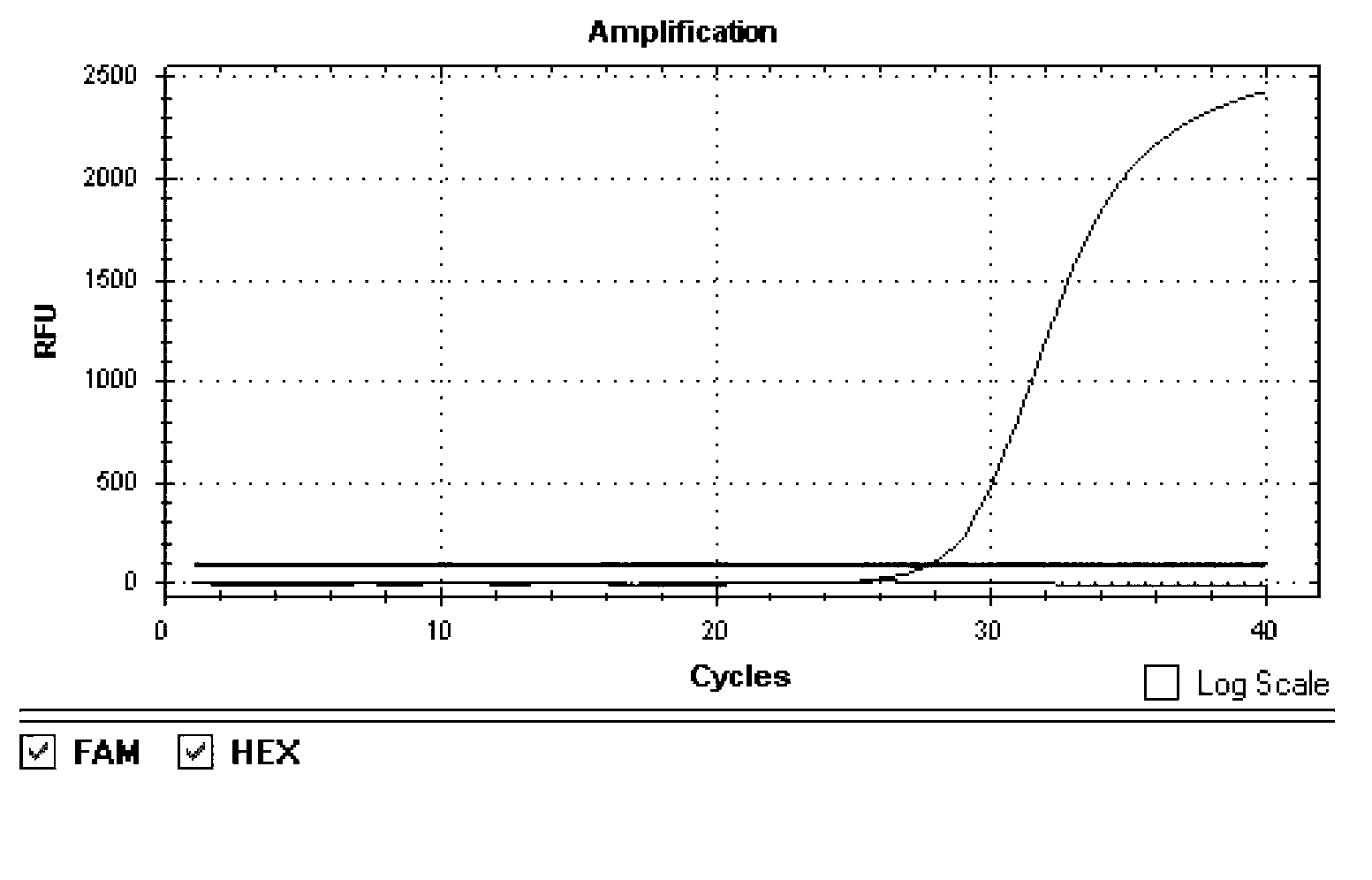
Image
Examples
Embodiment 1H1
[0060] Design and synthesis of embodiment 1 H1N1 / H5N1 double fluorescent PCR detection kit detection primer probe
[0061] The FAM fluorophore is labeled at the 5' of the H5N1 probe, and the HEX fluorophore is labeled at the 5' of the H1N1 probe. Synthesize primers and probes for H1N1 and H5N1.
[0062] H1N1
[0063] H1N1 upstream primer: 5'-CACCAGTCCACGATTGCAAT-3' (SEQ ID NO: 1)
[0064] H1N1 downstream primer: 5'-ATGGGAGGCTGGTGTTTATAGC-3' (SEQ ID NO: 2)
[0065] H1N1 probe: 5'HEX-CAACTTGTCAGACACCCAA-BHQ1-3' (SEQ ID NO: 3)
[0066] H5N1
[0067] H5N1 upstream primer: 5'-GGAATGGTAGATGGTTGGTATGG-3' (SEQ ID NO: 4)
[0068] H5N1 downstream primer: 5'-CTTTTGAGTGGATTCTTTGTCTGC-3' (SEQ ID NO: 5)
[0069] H5N1 probe: 5'FAM-ACCACCATAGCAACGAGCAGGGGA-BHQ1-3' (SEQ ID NO: 6)
Embodiment 2
[0070] The preparation of embodiment 2 kit dual fluorescence PCR detection mixed solution
[0071] H1N1 upstream primer 0.5 μl / test; H1N1 downstream primer 0.5 μl / test; H1N1 probe 0.1 μl / test; H5N1 upstream primer 0.5 μl / test; H5N1 downstream primer 0.5 μl / test; H5N1 probe 0.1 μl / test; RT -PCR MIX 12.5 μl / test; process water 4.3 μl / test, mix well to obtain dual fluorescent PCR detection mixture.
Embodiment 3H1
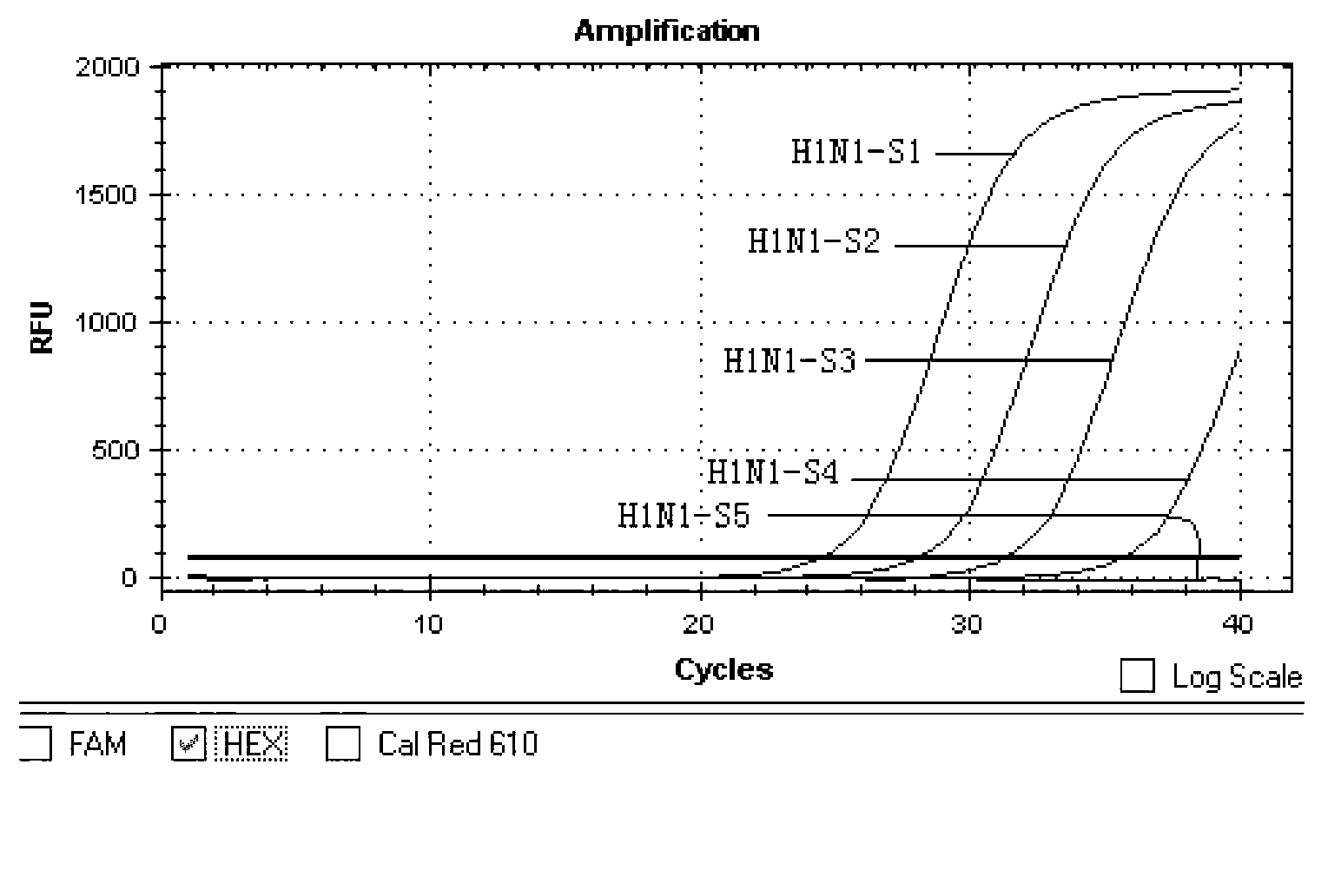
[0072] Sensitivity Analysis of Example 3 H1N1 / H5N1 Type Avian Influenza Virus Double Fluorescent PCR Detection Kit
[0073] 1. Experimental method
[0074] 1) Sample preparation:
[0075] The construction method of the H1N1 plasmid: PCR method is used to amplify the nucleic acid of the H1N1 virus sample to obtain the nucleic acid fragment containing the target amplification sequence of H1N1 (sequence shown in SEQ ID NO: 7). The primer sequences are shown in SEQ ID NO: 1-2 Show. After the amplified fragment was purified, it was cloned into the pMD8-T vector by TA, identified by sequencing, and the recombinant vector with correct sequencing result was transformed into DH5α and amplified to obtain the H1N1 plasmid.
[0076] The construction method of the H5N1 plasmid: PCR method is used to amplify the nucleic acid of the H5N1 virus sample to obtain a nucleic acid fragment containing the target amplification sequence of H5N1 (sequence shown in SEQ ID NO: 8). The primer sequences...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com