Method for continuously producing 3,4-dichloroaniline
A technology of dichloroaniline and dichloronitrobenzene, applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve problems such as complicated operation, large amount of hydrogen feed, hydrogen loss, etc., and achieve a control method The effect of simplicity, simplified process flow, and low hydrogen consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
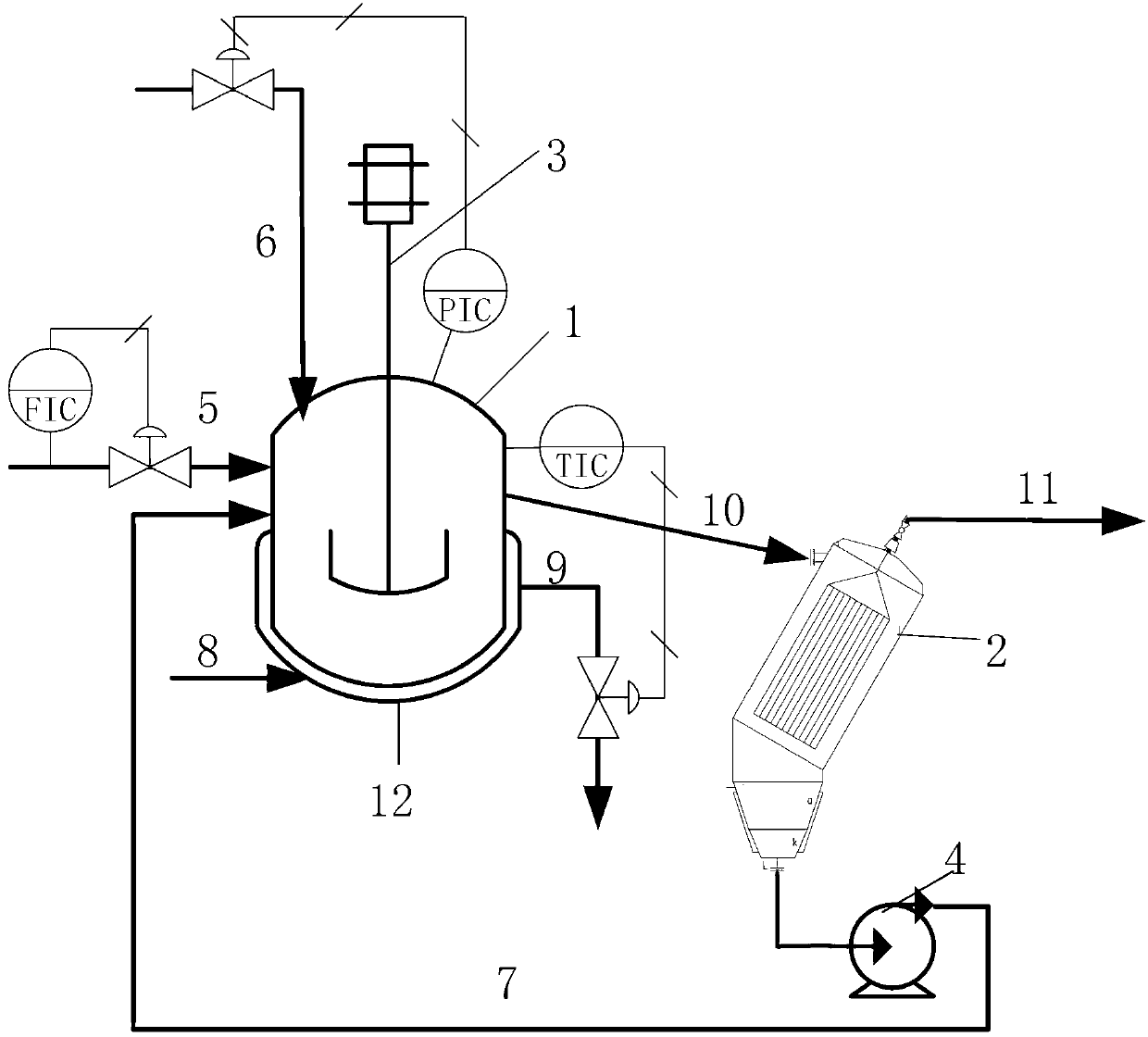
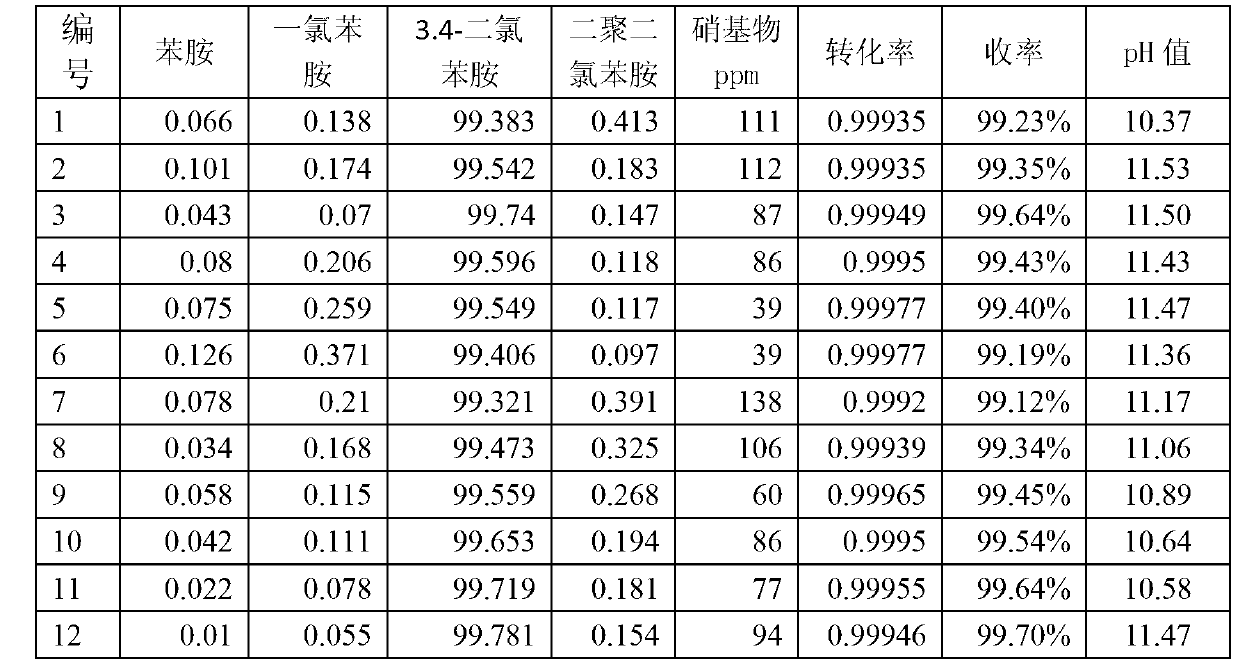
[0047] The stirred tank reactor 1 has a size of 1 L and is made of carbon steel. The reaction pressure is 1MPa, controlled by the flow of hydrogen; the temperature is 65°C, controlled by the flow of circulating water in the jacket 12 of the stirred tank reactor 1, the catalyst is Raney nickel, and the mass concentration is 1.3%. The stirred tank reactor 1 adopts a self-priming stirring paddle 3, and the stirring speed is 550 rpm. The dechlorination inhibitor is ethanolamine, and the mass concentration of the inhibitor in the kettle is 500ppm. The solvent was methanol, the feed was 3,4-dichloronitrobenzene in methanol solution, the mass concentration of 3,4-dichloronitrobenzene was 15%, and the feed rate was 10 mL / min. The effective volume of the reactor is 0.6L, and the residence time is 1h. The reaction product enters the inclined plate separator 2 through overflow, and the length of the straight plates in the inclined plate separator 2 is 0.2m, the width is 0.07m, the plat...
Embodiment 2
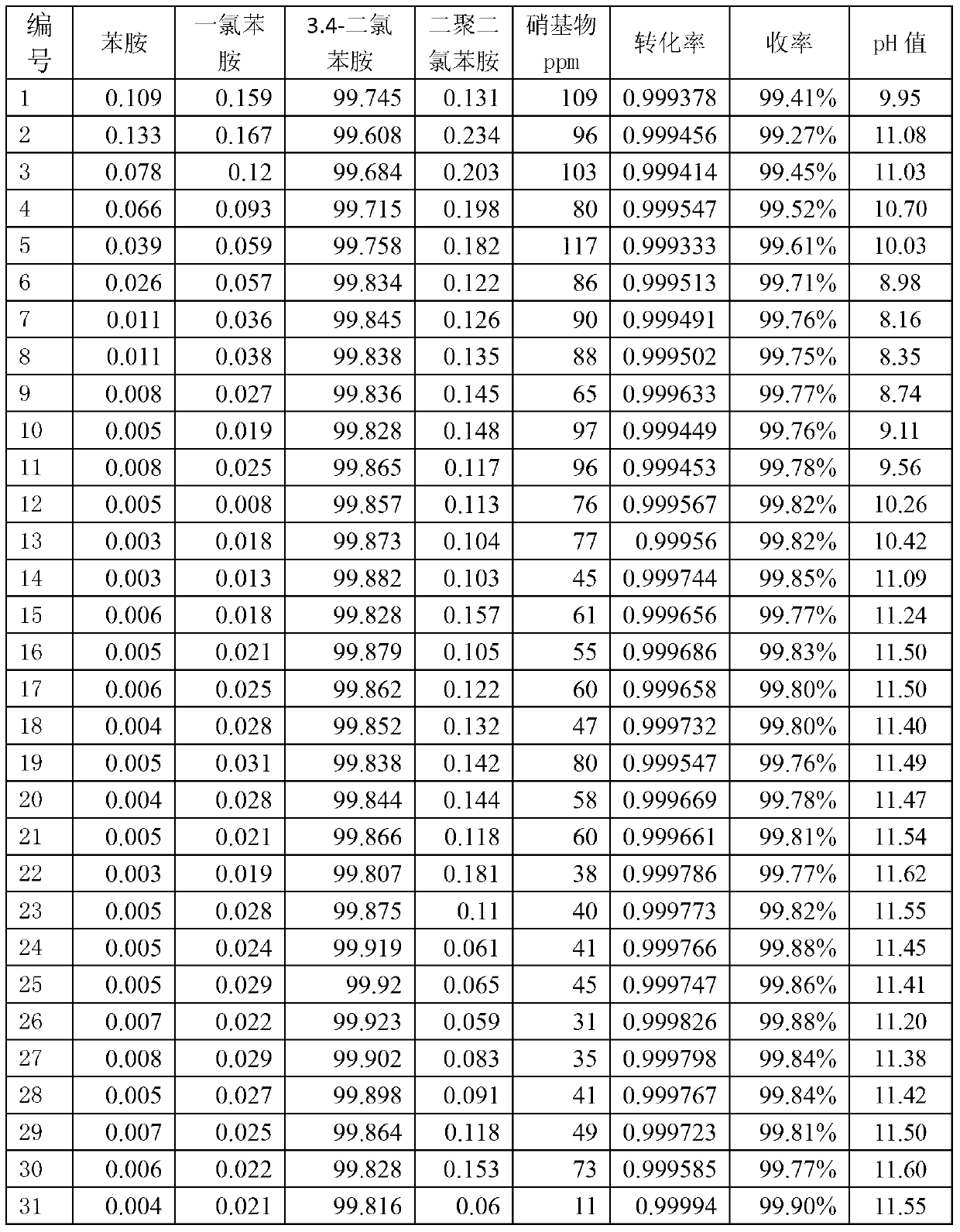
[0053] The stirred tank reactor 1 has a size of 1 L and is made of carbon steel. The reaction pressure is 0.8MPa, controlled by the flow of hydrogen; the temperature is 100°C, controlled by the flow of circulating water in the jacket 12 of the stirred tank reactor 1, and the catalyst is a diatomite nickel catalyst with a mass concentration of 2%. The stirred tank reactor 1 adopts a self-priming stirring paddle 3, and the stirring speed is 800 rpm. The dechlorination inhibitor is sodium sulfite, and the mass concentration of the inhibitor in the kettle is 800ppm. The solvent is ethanol, and the feed is an ethanol solution of 3,4-dichloronitrobenzene, the mass concentration of 3,4-dichloronitrobenzene is 5%, and the feed rate is 6.7mL / min. The effective volume of the reactor is 0.6L, and the residence time is 1.5h. The reaction product enters the inclined plate separator 2 through overflow, and the length of the straight plate in the inclined plate separator 2 is 0.1m, the wid...
Embodiment 3
[0057] The size of the stirred tank reactor 1 is 1m 3 , the material is carbon steel. The reaction pressure is 1.2MPa, controlled by the flow of hydrogen; the temperature is 90°C, controlled by the flow of circulating water in the jacket 12 of the stirred tank reactor 1, and the catalyst is activated carbon nickel catalyst with a mass concentration of 1.8%. The stirred tank reactor 1 adopts a self-priming stirring paddle 3, and the stirring speed is 200 rpm. The dechlorination inhibitor is thiourea, and the mass concentration of the inhibitor in the kettle is 1000ppm. Solvent adopts ethylene glycol, and feed is the ethylene glycol solution of 3,4-dichloronitrobenzene, and the mass concentration of 3,4-dichloronitrobenzene is 12%, and feed rate is 0.3m 3 / h. The effective volume of the reactor is 0.6m 3 , the residence time is 2h. Reaction product enters inclined plate separator 2 by overflowing, and the straight plate length in inclined plate separator 2 is 1m, and width ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| width | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com