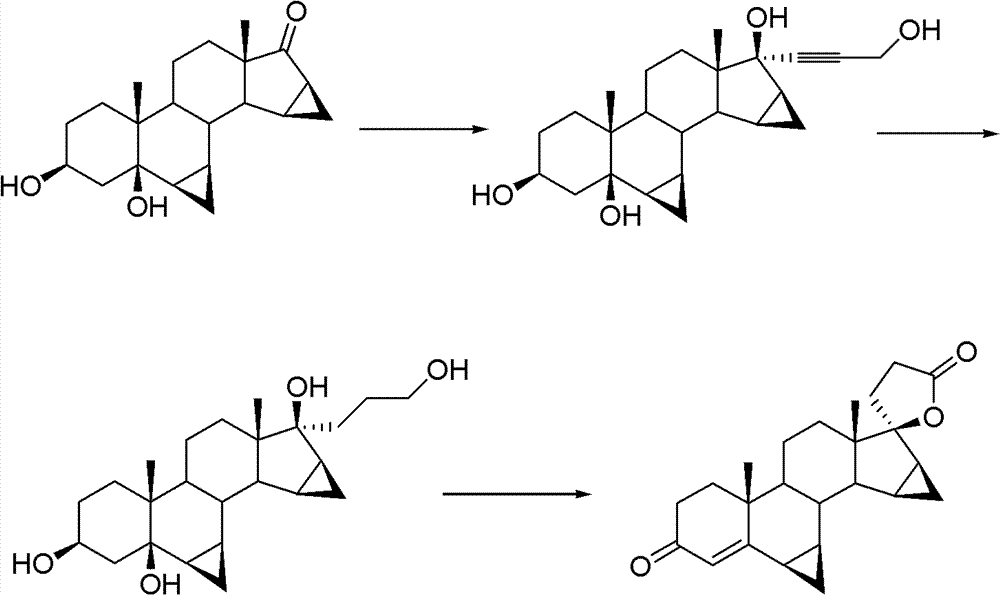
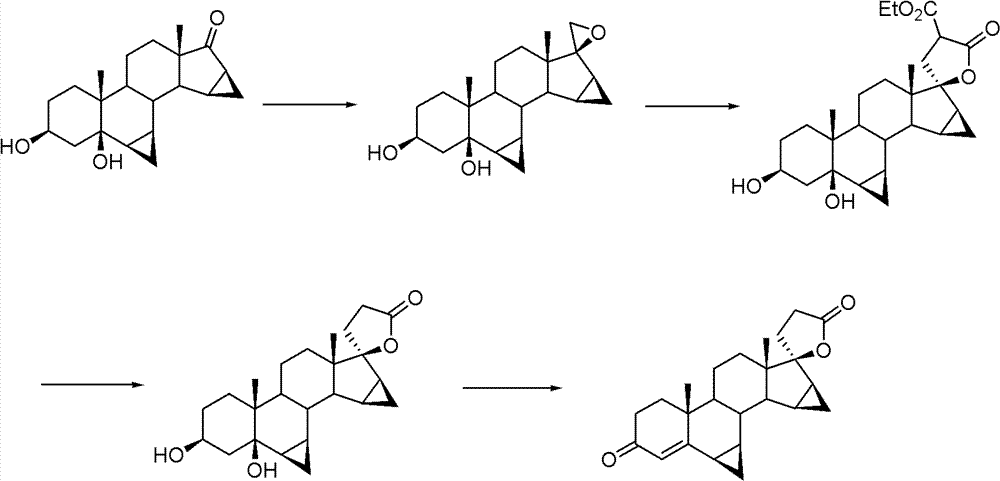
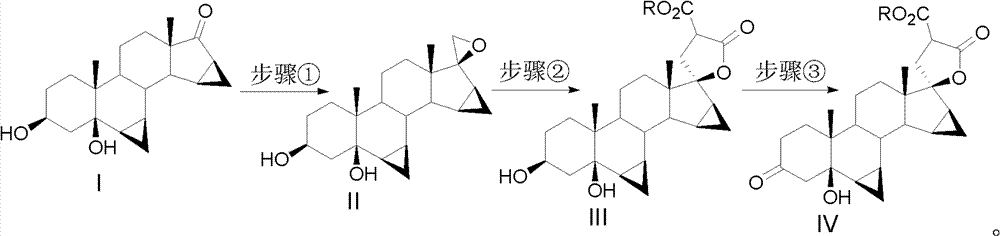
Drospirenone intermediate, and preparation method thereof, application thereof in preparation of drospirenone
An intermediate, drospirenone technology, applied in the field of medicinal chemistry, can solve the problems of unsuitability for industrial production and high toxicity, and achieve the effects of simple preparation, stable quality, and reduced pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] One, preparation formula II compound
[0034] Heat 60mL dimethyl sulfoxide (DMSO), 40mL tetrahydrofuran (THF), and 10g potassium tert-butoxide to 60°C for 30 minutes, then cool to 0~-10°C, add 16g trimethyl bromide sulfide, and Stir at 0°C for 30 minutes, then add 10 g of compound of formula I, react at 0°C for 1 hour, monitor the completion of the reaction by TLC, crystallize in ice water, filter, and dry to obtain 10.3 g of compound of formula II.
[0035] TLC monitoring conditions are: cyclohexane: ethyl acetate = 1:2.
[0036] 2. Compound of formula III: 3β, 5β-dihydroxy-6β, 7β, 15β, 16β-dimethylene-5β-androsta-21, 17-carboxylate-21-ethyl formate
[0037] Dissolve 12g of sodium ethoxide in 100mL of absolute ethanol, heat to 30-35°C, add 30mL of condensing agent diethyl malonate, raise the temperature to an internal temperature of 35-40°C, stir for 10 minutes, then add 10g of the compound of formula II , heated to reflux, reflux reaction for 3 hours, TLC monitored ...
Embodiment 2
[0049] One, preparation formula II compound
[0050] As described in Example 1.
[0051] 2. Compound of formula III: 3β, 5β-dihydroxy-6β, 7β, 15β, 16β-dimethylene-5β-androsta-21, 17-carboxylide-21-formic acid
[0052] Dissolve 12g of sodium ethoxide in 100mL of absolute ethanol, heat to 30-35°C, add 22mL of condensing agent monoethyl malonate, raise the temperature to an internal temperature of 35-40°C, stir for 10 minutes, then add 10g of the compound of formula II , heated to reflux, reflux reaction for 3 hours, TLC monitored the completion of the reaction, cooled to room temperature, filtered, adjusted the pH of the filtrate to 6-7, crystallized in ice water, filtered, and dried to obtain 11.1 grams of the compound of formula III: 3β, 5β -Dihydroxy-6β, 7β, 15β, 16β-dimethylene-5β-androst-21,17-carboxylactone-21-carboxylic acid.
[0053] TLC monitoring conditions are: cyclohexane: ethyl acetate = 1:2.
[0054] 3. Compound of formula IV: 6β, 7β, 15β, 16β-dimethylene-5β-hyd...
Embodiment 3
[0063] One, preparation formula II compound
[0064] As described in Example 1.
[0065] 2. Compound of formula III: 3β, 5β-dihydroxy-6β, 7β, 15β, 16β-dimethylene-5β-androsta-21, 17-carboxylate-21-sodium formate
[0066] Dissolve 12g of sodium ethoxide in 100mL of absolute ethanol, heat to 30-35°C, add 33g of condensing agent ethyl malonate sodium salt, raise the temperature to an internal temperature of 35-40°C, stir for 10 minutes, then add 10g of formula II Compound, heated to reflux, reflux reaction for 3 hours, TLC monitored the completion of the reaction, cooled to room temperature, filtered, adjusted the pH of the filtrate to 6-7, crystallized in ice water, filtered, and dried to obtain 12 grams of oil: 3β, 5β -dihydroxy-6β, 7β, 15β, 16β-dimethylene-5β-androst-21,17-carboxylactone-21-carboxylic acid; the resulting oil was dissolved in 120 mL of methanol and cooled to -5 ~0°C, add 1.5g sodium methoxide, stir at -5~0°C for 2 hours, suction filter and dry to obtain 10.5g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com