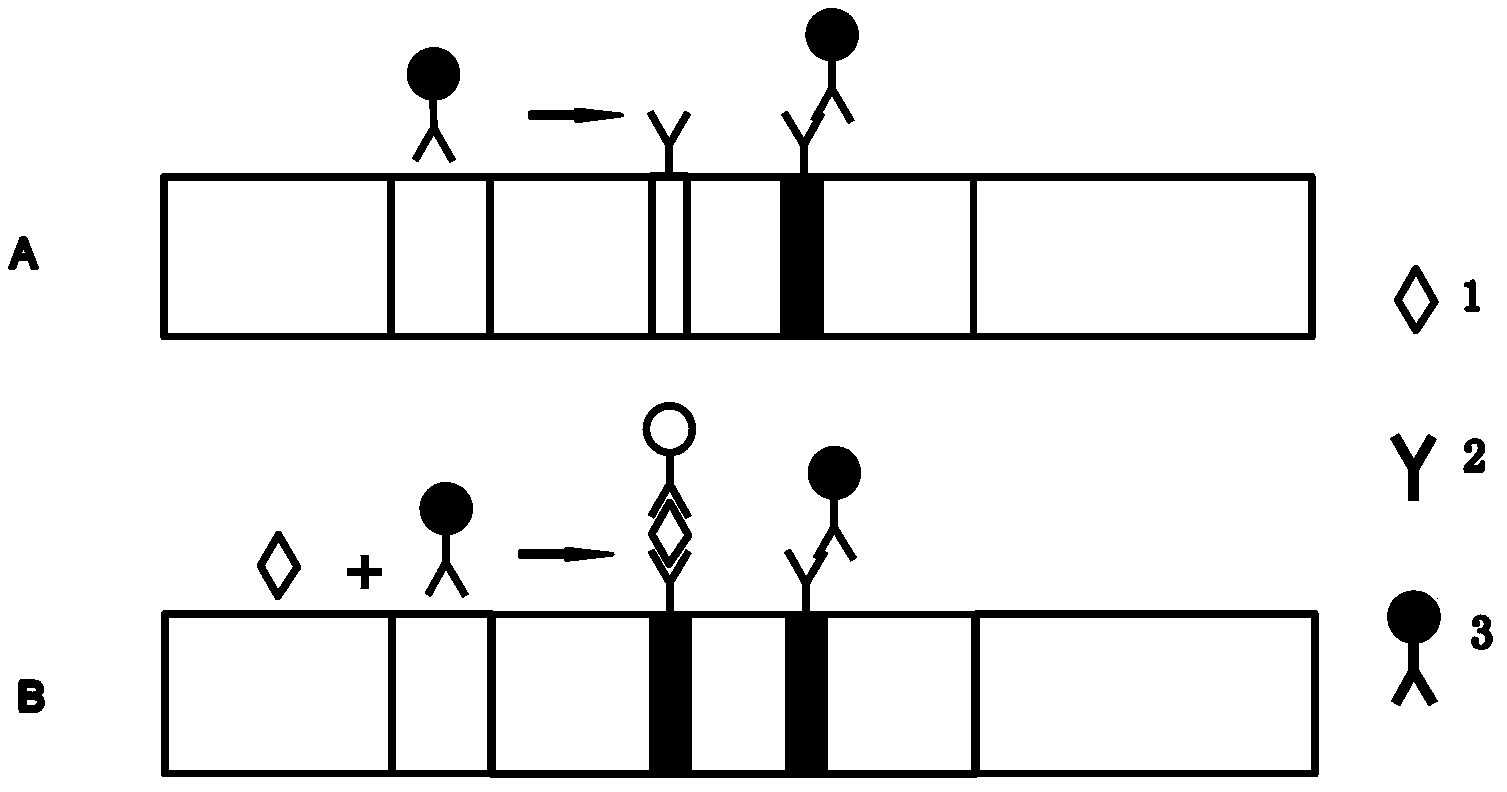
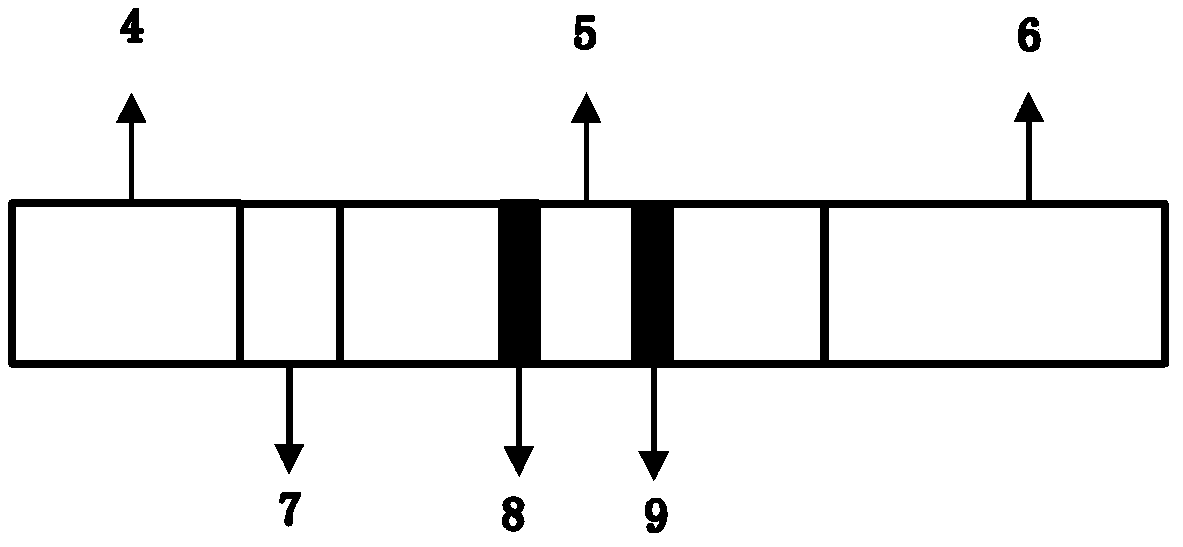
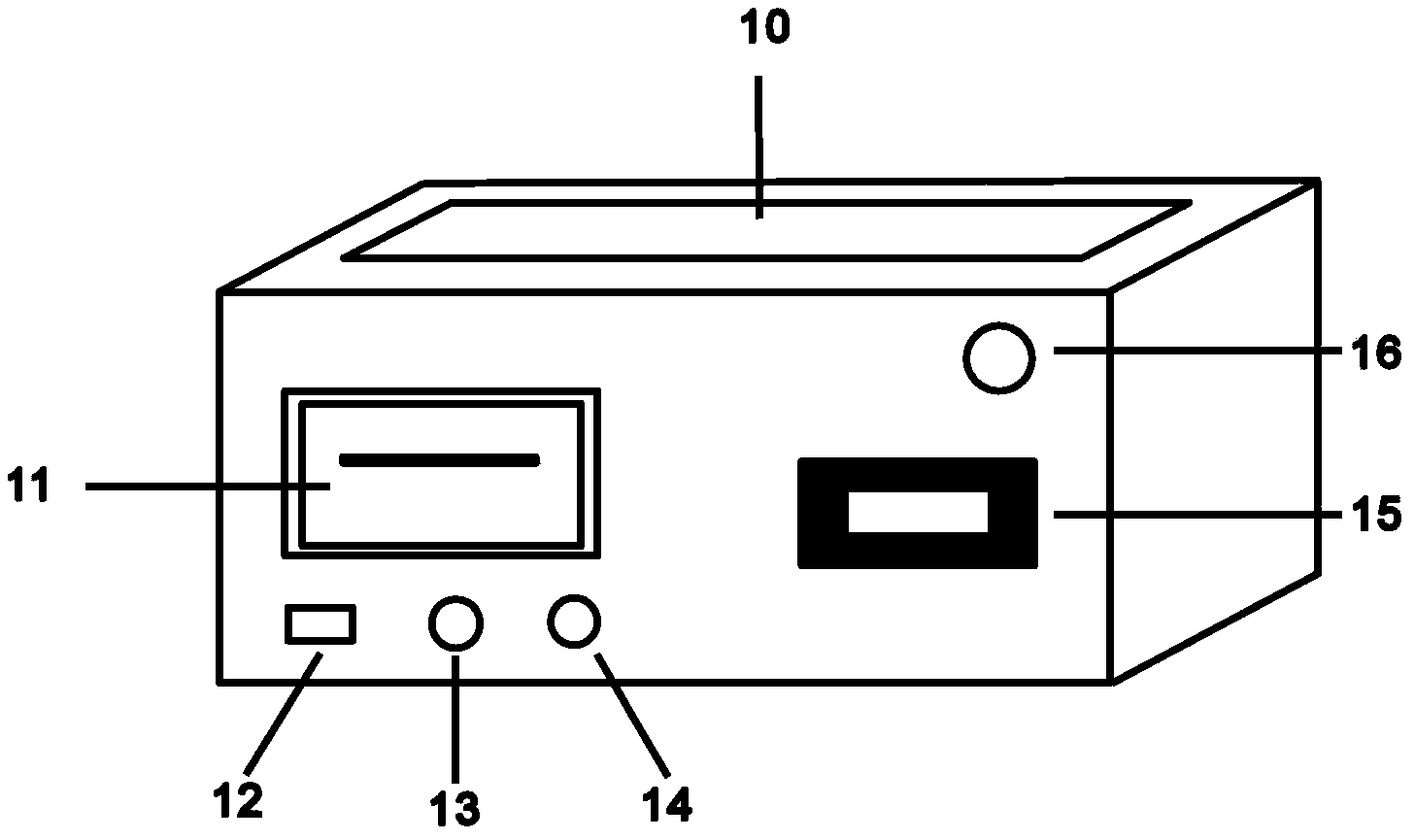
Method and device for rapidly and quantitatively detecting tumor marker with immunochromatography test strip marked by quantum dots
A technology of tumor markers and immunochromatography, which is applied in the field of rapid quantitative detection of tumor markers and devices using quantum dot-labeled immunochromatography test strips, which can solve the problem that quantitative detection cannot be completed, and the color of the detection strip is light and cannot be detected by naked eyes. Identification and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Preparation and assembly process of quantum dot-labeled immunochromatographic test strips. The quantum dots used are CdZnSe quantum dots, and the emission wavelength is 530nm; the tumor marker antibody used is β-hCG monoclonal antibody, and the ratio of quantum dots to antibodies is 30:2; the chemical coupling agent used is ethyl-( 3-Dimethylpropyl) carbodiimide hydrochloride (EDC), the ratio of quantum dots to EDC is 12:1.
[0053] 1. Antibodies related to tumor markers coupled with quantum dots
[0054] (1) Weigh 0.5mg EDC, dissolve it in 1mL deionized water, and set aside; take 6mg quantum dots and disperse them in 1mL PBS buffer, set aside; take 0.4mgβ-hCG monoclonal antibody into the quantum dot solution, vortex Rotate at a low speed on the instrument to mix evenly, add EDC aqueous solution to the quantum dot solution during the vortex process, place the above mixed solution on a rotating mixing rack, and react at room temperature for 2 hours.
[0055] (2) Purify...
Embodiment 2
[0070] Preparation and assembly process of quantum dot-labeled immunochromatographic test strips. The quantum dots used are CdTe / ZnSe quantum dots, the emission wavelength is 634nm, the tumor marker antibody used is CEA monoclonal antibody, the ratio of quantum dots to antibodies is 9:2, and the chemical coupling agent used is ethyl-( 3-Dimethylpropyl) carbodiimide hydrochloride (EDC), the ratio of quantum dots to EDC is 16:3.
[0071] 1. Antibodies related to tumor markers coupled with quantum dots
[0072] (1) Weigh 0.9mg EDC, dissolve it in 1mL deionized water, and set aside; take 9mg quantum dots and disperse them in 1mL PBS buffer, set aside; take 2mg CEA monoclonal antibody and add them to the quantum dot solution, and place on a vortex instrument Rotate at a low speed to make it evenly mixed, and add EDC aqueous solution to the quantum dot solution during the vortex process. The above mixed solution was placed on a rotating mixing rack and reacted at room temperature ...
Embodiment 3
[0088] Preparation and assembly process of quantum dot-labeled immunochromatographic test strips. The quantum dots used are ZnCuInS 3 Quantum dots, the emission wavelength is 720nm; the tumor marker antibody used is AFP monoclonal antibody, the ratio of quantum dots to antibody mass fraction is 4:1; the chemical coupling agent used is ethyl-(3-dimethylpropyl) Carbodiimide hydrochloric acid (EDC), the mass fraction ratio of quantum dots to EDC is 6:1.
[0089] 1. Antibodies related to tumor markers coupled with quantum dots
[0090] (1) Weigh 2 mg of EDC, dissolve in 1 mL of deionized water, and set aside; take 12 mg of quantum dots and disperse them in 1 mL of PBS buffer, and set aside; take 3 mg of AFP monoclonal antibody into the quantum dot solution, and rotate at a low speed on a vortex instrument Make it mix well, and add EDC aqueous solution to the quantum dot solution during the vortex process. The above mixed solution was placed on a rotating mixing rack and reacted...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com