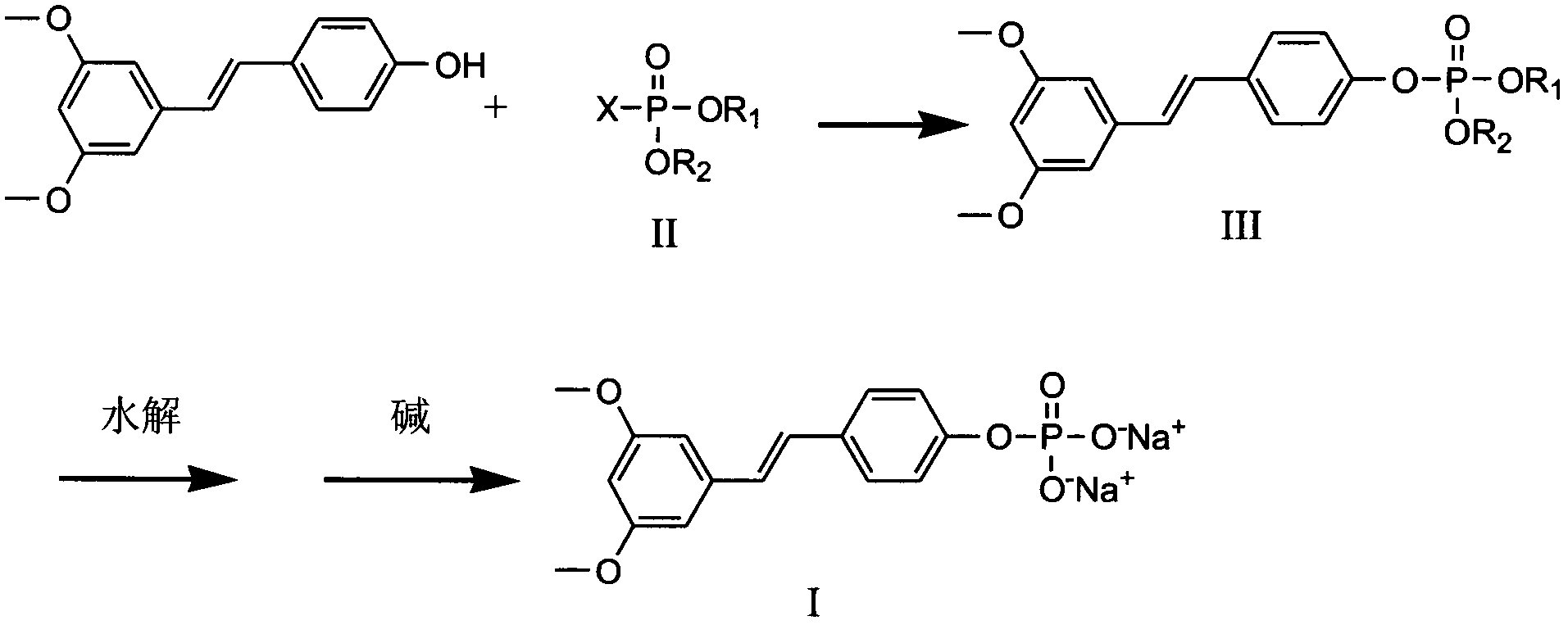
Pterostilbene phosphate disodium salt synthesis method
A technology of pterostilbene phosphate disodium salt and pterostilbene phosphate, which is applied in the field of synthesis of water-soluble pterostilbene derivative pterostilbene phosphate disodium salt, can solve the problems of difficult quality control, many reaction by-products, and product yield. Low-level problems, to achieve the effect of short response time, simple operation and good quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Under nitrogen protection, 2.56 g (0.01 mol) of pterostilbene, 0.06 g (0.0005 mol) of 4-dimethylaminopyridine and 1.42 g (0.011 mol) of diisopropylethylamine were dissolved in 20 ml of dichloromethane, at 20 °C, a solution of 2.51 g (0.011 mol) of di-(tert-butoxy)-phosphoryl chloride and 10 ml of dichloromethane was added dropwise. After the dropwise addition, keep the temperature and stir the reaction, monitor the reaction process with thin-layer chromatography, until the raw material point disappears, the reaction time is 0.5 hour, the reaction solution is poured into 50ml ice water, the organic layer is separated, and the water layer is refilled with 20ml diethyl alcohol. Extract once with methyl chloride, combine the organic layers, wash with water, dry, and evaporate to dryness to obtain a yield of 94% oil pterostilbene-di-(tert-butoxy)-phosphate 4.07g, and put it into 50ml of pre-dried hydrogen chloride into saturated methanol, stirred at room temperature for 24 h...
Embodiment 2
[0028] Under nitrogen protection, dissolve pterostilbene 2.56g (0.01moi), 4-dimethylaminopyridine 0.06g (0.0005mol) and diisopropylethylamine 1.428 (0.011mol) in 20ml of dichloromethane, at 30°C A solution of 2.45 g (0.011 mol) of bis-(2-cyanoethoxy)-phosphoryl chloride and 10 ml of dichloromethane was added dropwise. After the dropwise addition is completed, keep the temperature and stir the reaction, monitor the reaction process with thin layer chromatography, until the raw material point disappears, the reaction time is 0.8 hours, the reaction solution is poured into 50ml of ice water, the organic layer is separated, and the water layer is re-used with 20ml of two Methyl chloride was extracted once, the organic layers were combined, washed with water, dried, and evaporated to dryness to obtain a yield of 93% oil pterostilbene-bis-(2-cyanoethoxy)-phosphate 4.45g, which was dropped into 25ml of methanol, Add NaOH-methanol solution dropwise under stirring until the pH is 8-10,...
Embodiment 3
[0030] Under nitrogen protection, dissolve pterostilbene 2.56g (0.01mol), 4-dimethylaminopyridine 0.068 (0.0005mol) and diisopropylethylamine 1.42g (0.011mol) in 20ml of chloroform, at 20°C A solution of 6.61 g (0.011 mol) of bis-(triphenylmethoxy)-phosphoryl chloride and 10 ml of chloroform was added dropwise. After the dropwise addition is completed, keep the temperature and stir the reaction, monitor the reaction process with thin layer chromatography, until the raw material point disappears, the reaction time is 0.5 hour, the reaction solution is poured into 50ml ice water, separate the organic layer, and the water layer is washed with 20ml three Chloromethane was extracted once, and the organic layers were combined, washed with water, dried, and evaporated to dryness to obtain a yield of 89% oil pterostilbene-bis-(triphenylmethoxy)-phosphate 7.92g, and put it into 50ml that had been passed through in advance. Dry hydrogen chloride into saturated methanol, stir at room tem...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com