Boron-doped lithium-rich anode material for lithium ion batteries and preparation method of material
一种富锂正极材料、锂离子电池的技术,应用在锂离子电池材料和电化学领域,能够解决循环容量骤减、循环性能一般、限制商业化应用等问题,达到振实密度提升、产物颗粒均匀细小、稳定性提高的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
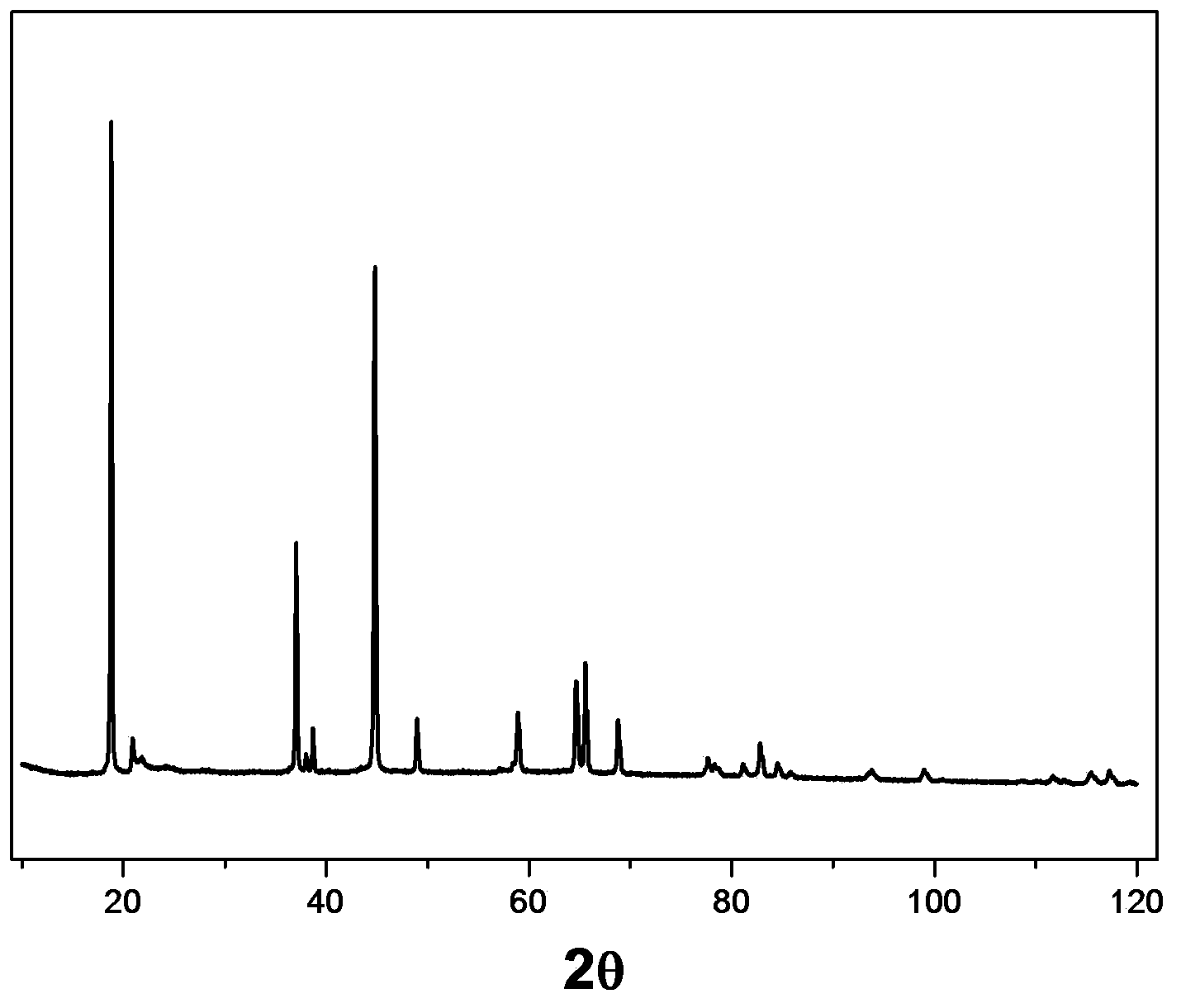
[0034] Embodiment 1 sol-gel method synthesizes the ternary lithium-rich material Li[Li] doped with boron 2%. 0.2 mn 0.534 Ni 0.123 co 0.123 B 0.02 ]O 2
[0035] Take 3.215g of lithium acetate, 0.788g of nickel acetate, 3.229g of manganese acetate, 0.789g of cobalt acetate, 0.031g of boric acid, 12.615g of citric acid and 4.966g of ethylene glycol and dissolve them in 350ml of deionized water, stir and mix well, Put it in a pear-shaped bottle, and then rotate it in a rotary evaporator, the temperature is set at 80° C., and the rotation speed is 55 rpm. After steaming into a gel, place it in a vacuum oven at 150°C for more than 5 hours. Take out the dried gel, grind it and place it in a tube furnace for pre-calcination at 450°C for 4 hours, followed by calcination at 900°C for 15 hours to obtain the target product—a ternary lithium-rich material doped with 2% boron Li[Li 0.2 mn 0.534 Ni 0.123 co 0.123 B 0.02 ]O 2 .
[0036] The scanning electron microscope image of...
Embodiment 2
[0039] Embodiment 2 sol-gel method synthesizes the ternary lithium-rich material Li[Li] doped with boron 4% 0.2 mn 0.52 Ni 0.12 co 0.12 B 0.04 ]O 2
[0040] Take 3.215g of lithium acetate, 0.747g of nickel acetate, 3.191g of manganese acetate, 0.748g of cobalt acetate, 0.062g of boric acid, 12.610g of citric acid and 4.969g of ethylene glycol and dissolve them in 350ml of deionized water, stir and mix well. Put it in a pear-shaped bottle, and then rotate it in a rotary evaporator, the temperature is set at 80° C., and the rotation speed is 55 rpm. After steaming into a gel, place it in a vacuum oven at 150°C for more than 5 hours. The dried gel was taken out, crushed and placed in a tube furnace for pre-calcination at 450°C for 4 hours, followed by calcination at 900°C for 15 hours to obtain the target product.
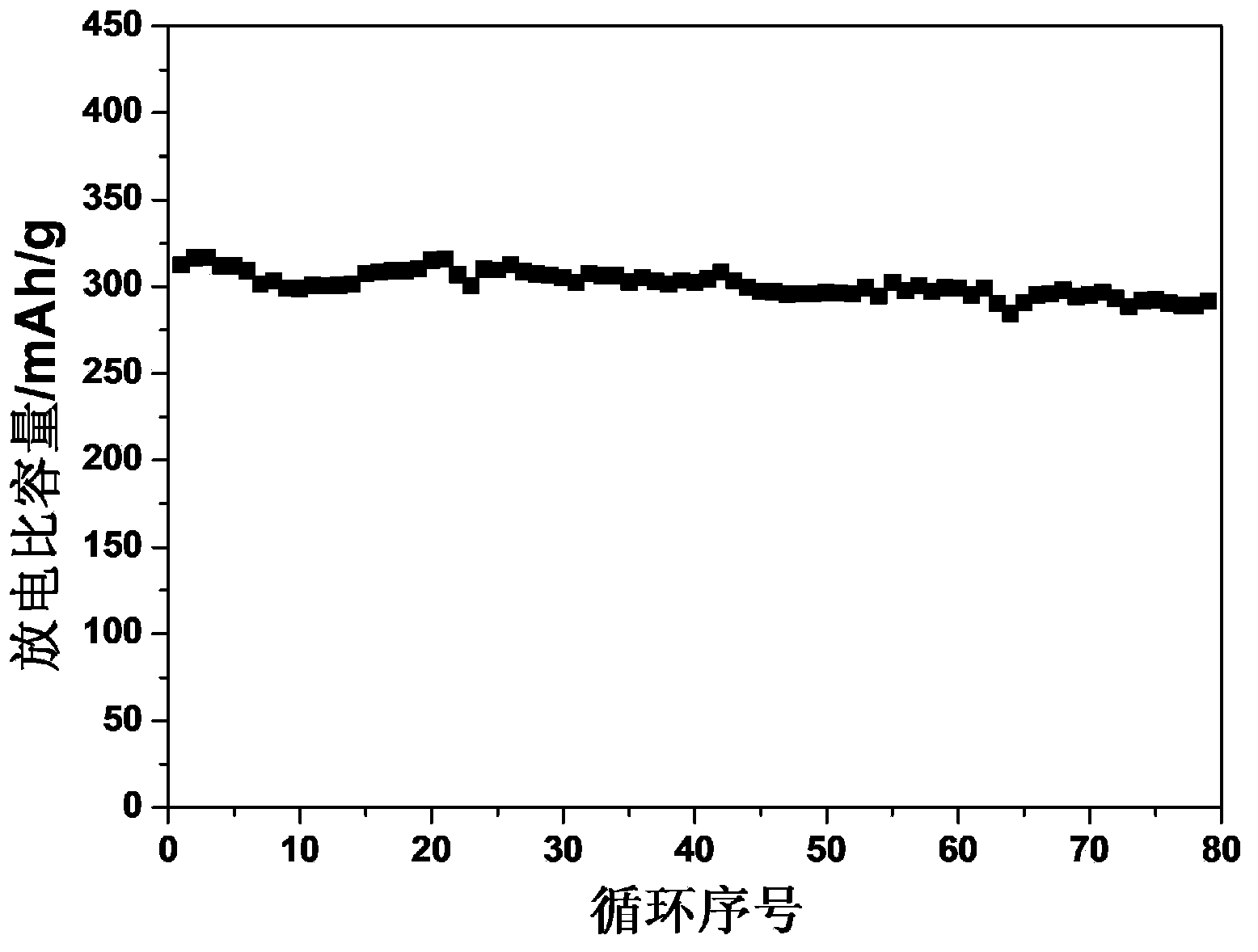
[0041] Mix the target product with carbon black and PVDF at a mass ratio of 8:1:1, grind evenly with N-methylpyrrolidone as a solvent, then coat it on an alumi...
Embodiment 3
[0043] Example 3 The sol-gel method synthesizes the ternary lithium-rich material Li[Li] doped with boron 8%. 0.2 mn 0.514 Ni 0.103 co 0.103 B 0.08 ]O 2
[0044] Take 3.221g of lithium acetate, 0.667g of nickel acetate, 3.107g of manganese acetate, 0.665g of cobalt acetate, 0.125g of boric acid, 12.626g of citric acid and 4.966g of ethylene glycol and dissolve them in 350ml of deionized water, stir and mix evenly. Put it in a pear-shaped bottle, and then rotate it in a rotary evaporator, the temperature is set at 80° C., and the rotation speed is 55 rpm. After steaming into a gel, place it in a vacuum oven at 150°C for more than 5 hours. The dried gel was taken out, crushed and placed in a tube furnace for pre-calcination at 450°C for 4 hours, followed by calcination at 900°C for 15 hours to obtain the target product.
[0045] Mix the target product with carbon black and PVDF at a mass ratio of 8:1:1, grind evenly with N-methylpyrrolidone as a solvent, then coat it on a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| composition ratio | aaaaa | aaaaa |
| actual density | aaaaa | aaaaa |
| actual density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com