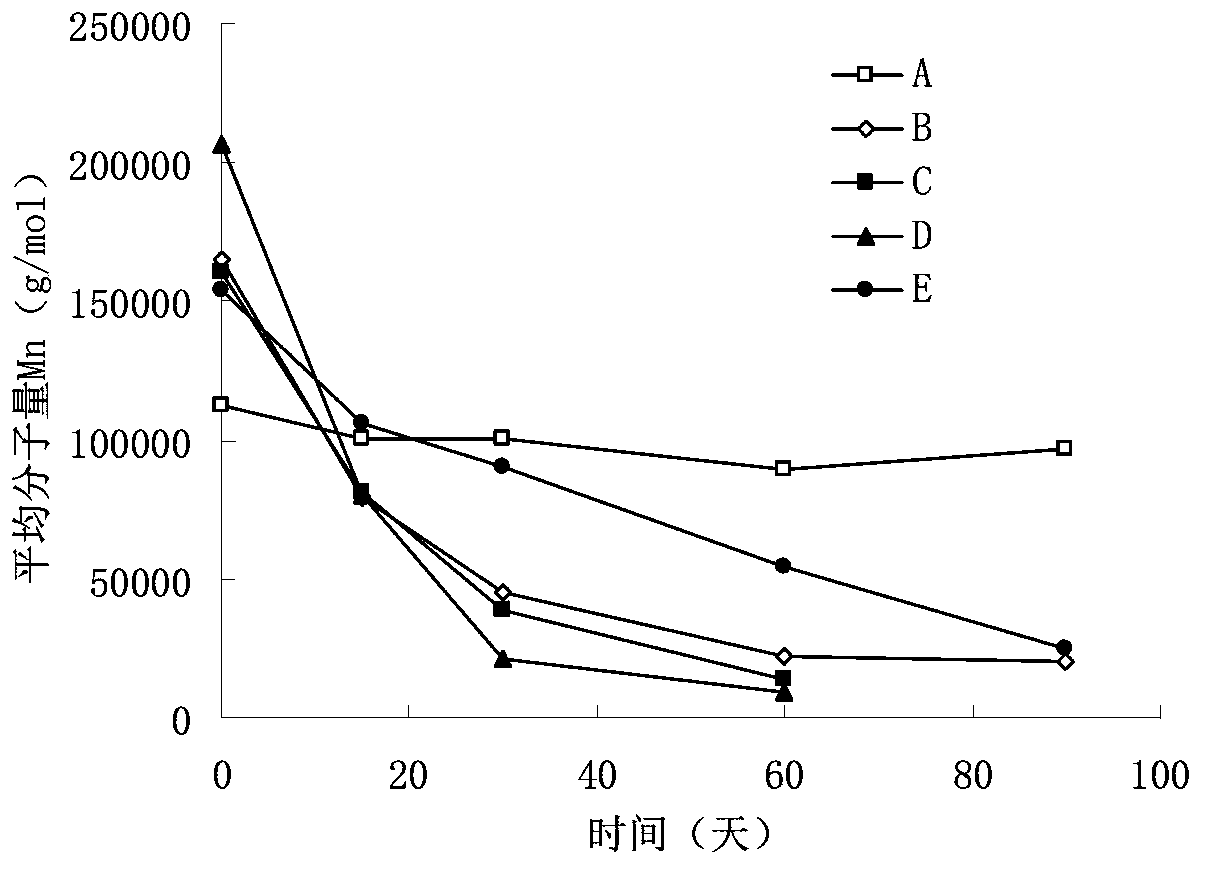
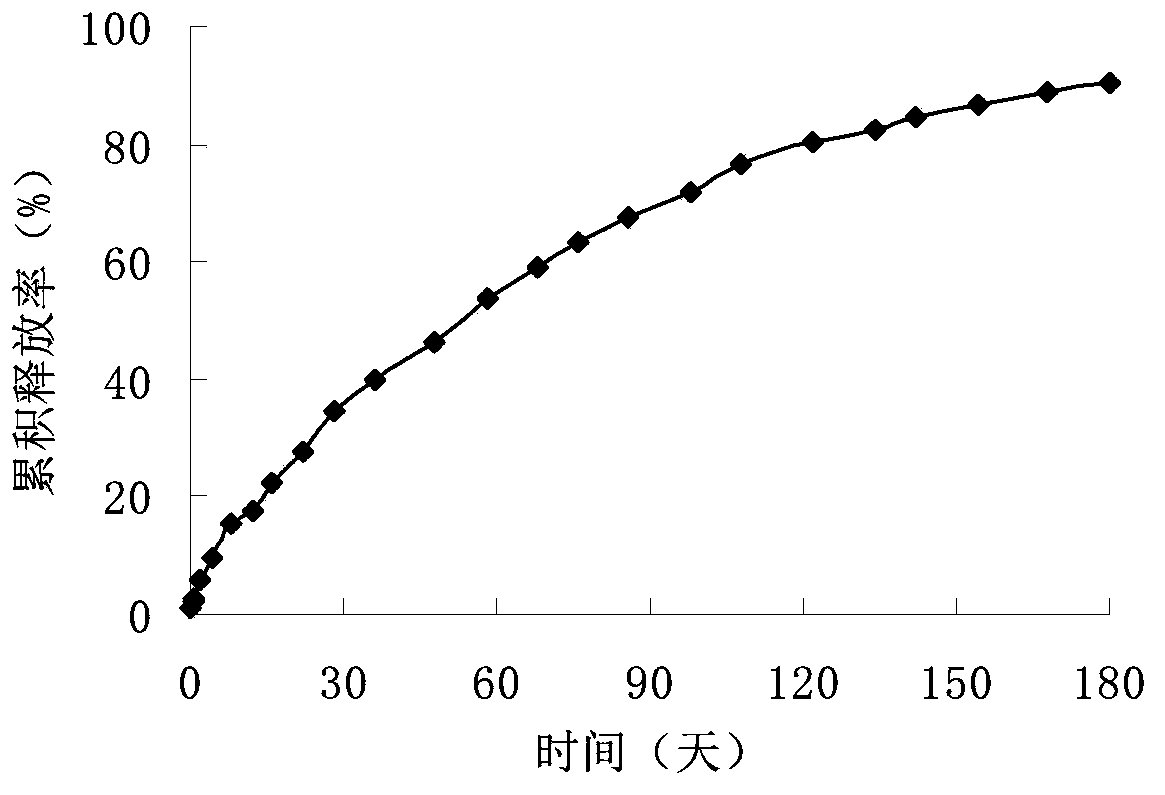
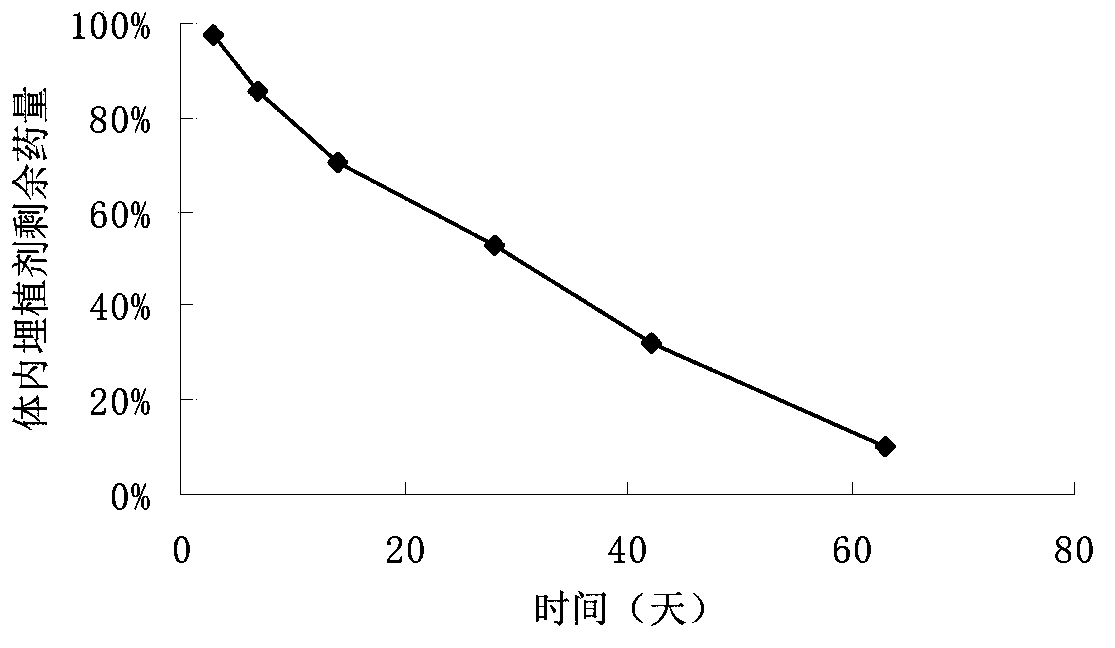
In-situ gel injection implant
A technology for injecting implants and in-situ gels, which is applied in the field of in-situ gel injection implants, can solve the problems of increased side effects, inconvenience, and repeated disease, and achieve the effect of simple preparation and convenient use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Preparation of levonorgestrel female contraceptive in situ gel injection implant
[0043] prescription:
[0044] Each g of levonorgestrel (LNG) in situ gel injection implant contains:
[0045] P(TMC-LLA): 174mg
[0046] LNG: 6mg
[0047] NMP: 820mg
[0048] Among them, the specification of P(TMC-LLA) is TMC:LLA=59:41 (molar ratio), and the weight average molecular weight (Mw) is 346,125.
[0049] After the P(TMC-LLA) was cut into pieces, weighed the prescription amount and added a certain amount of NMP to mix evenly, and stirred overnight at 37°C until a transparent and uniform solution was obtained. The resulting solution was heated at 65°C to remove air bubbles therein. After levonorgestrel is crushed through a 100-mesh sieve, the prescribed amount of levonorgestrel powder is added and dissolved in the above solution, stirred to obtain a transparent and uniform solution, and levonorgestrel for female contraception is obtained after γ-ray irradiation disinfection ...
Embodiment 2
[0051] Preparation of Testosterone Undecanoate Male Contraceptive In situ Gel Injection Implant
[0052] prescription:
[0053] Each g of testosterone undecanoate (TU) in situ gel injection implant contains:
[0054] P(CL-DLLA): 187.5mg
[0055] TU: 187.5 mg
[0056] NMP: 625mg
[0057] Among them, the specification of P(CL-DLLA) is CL:DLLA=80:20 (molar ratio), Mw=70357.
[0058] After the P(CL-DLLA) was cut into pieces, weighed the prescription amount and added a certain amount of NMP to mix evenly, and stirred overnight at 37°C until a transparent and uniform solution was obtained. The resulting solution was heated at 65°C to remove air bubbles therein. After testosterone undecanoate is crushed through a 100-mesh sieve, the prescribed amount of testosterone undecanoate powder is added and dissolved in the above solution, stirred to obtain a transparent and uniform solution, sterilized by γ-ray irradiation to obtain testosterone undecanoate in situ gel injection Implant...
Embodiment 3
[0060] Preparation of estradiol in situ gel injection implant
[0061] prescription:
[0062] Each g of estradiol in situ gel injection implant contains:
[0063] PTMC: 240mg
[0064] Estradiol: 6mg
[0065] NMP: 750mg
[0066] Among them, PTMC, Mw=48578.
[0067] After the PTMC is shredded, weigh the prescription amount and add a certain amount of NMP to mix evenly, and stir overnight at 37°C until a transparent and uniform solution is obtained. The resulting solution was heated at 65°C to remove air bubbles therein. After estradiol is crushed through a 100-mesh sieve, the prescribed amount of estradiol powder is added and dissolved in the above solution, stirred to obtain a transparent and uniform solution, and γ-ray irradiation is used to sterilize to obtain an estradiol in situ gel injection implant .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com