Medicine composition for preventing and treating arthritis and application thereof
A composition and technology for arthritis, applied in the application field of medicine or health care products, can solve the problems of low immunity, long course of treatment, single curative effect of arthritis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
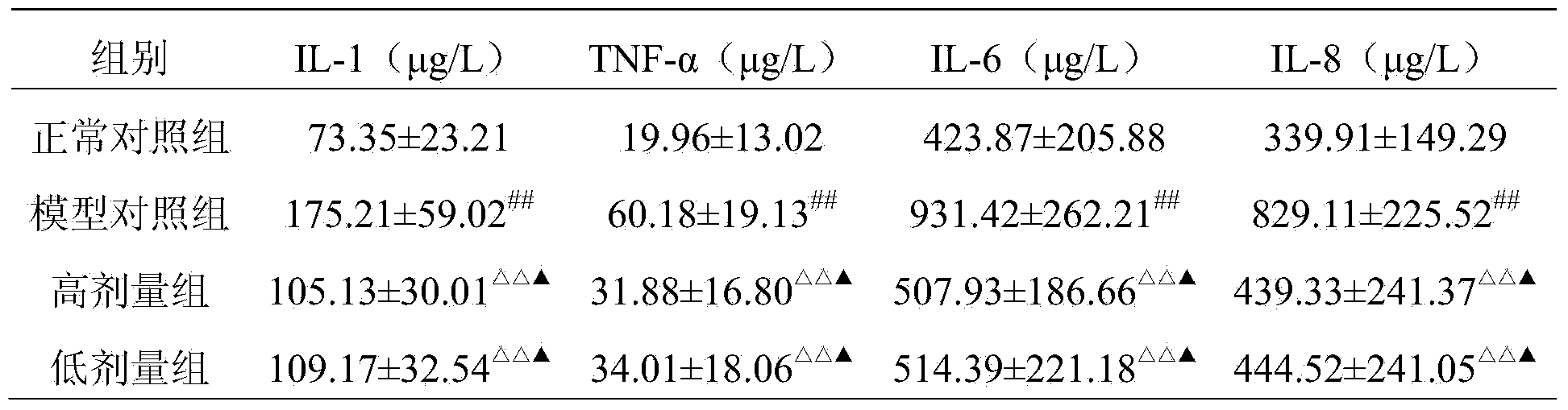
Embodiment 1
[0072] 1. Weigh 110g of glucosamine hydrochloride, 135g of biological calcium carbonate, 30g of Rhizoma Drynariae extract, 4.5g of casein phosphopeptide, 70g of chondroitin sulfate, and 46.5g of Epimedium extract, and pass through a 80-mesh sieve , qualified spare.
[0073] 2. Mix the substances described in step 1 uniformly to obtain the pharmaceutical composition of the present invention.
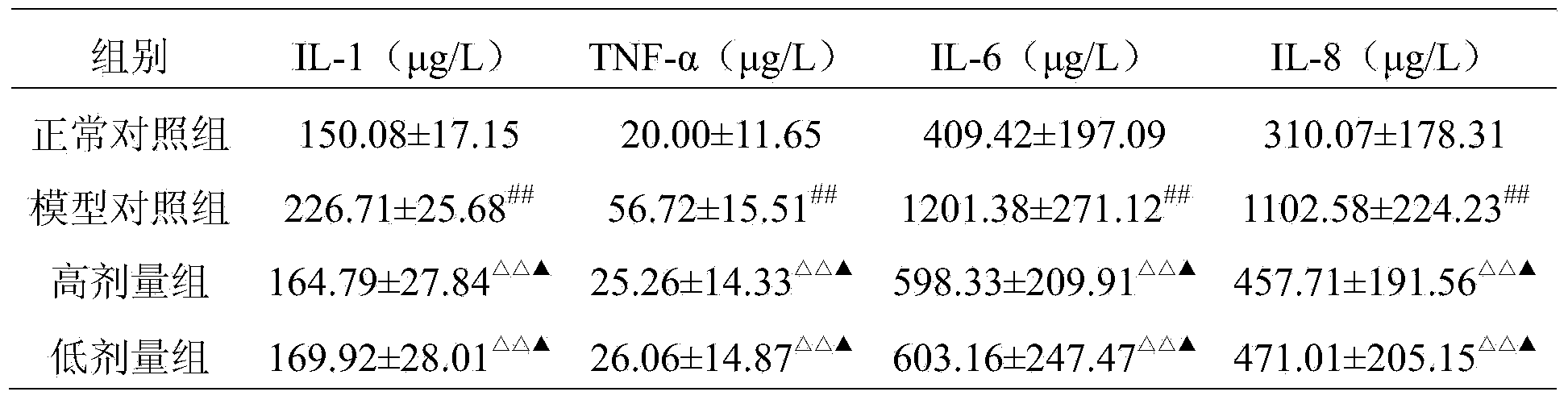
Embodiment 2
[0075] 1. Weigh 85g of glucosamine hydrochloride, 100g of biological calcium carbonate, 60g of Rhizoma drynaria extract, 5g of casein phosphopeptide, 45g of chondroitin sulfate, and 100g of Epimedium extract for bacterial inspection, pass through a 80-mesh sieve, and pass the test spare.
[0076] 2. Mix the substances described in step 1 uniformly to obtain the pharmaceutical composition of the present invention.
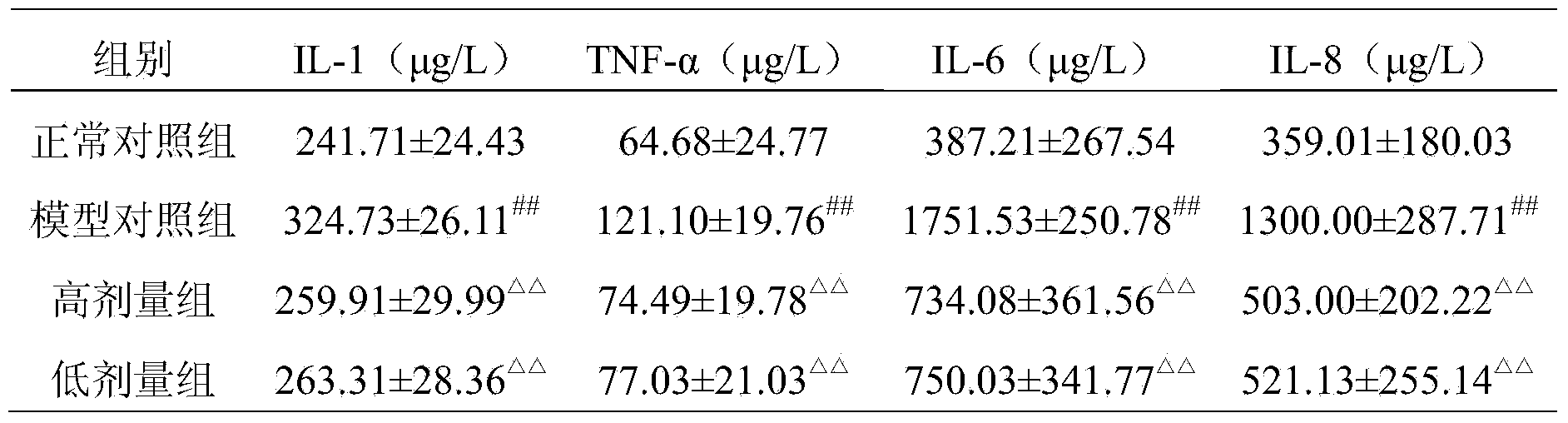
Embodiment 3
[0078] 1. Bacterial inspection of 90g of glucosamine hydrochloride, 115g of biological calcium carbonate, 50g of drynaria extract, 6g of casein phosphopeptide, 54g of chondroitin sulfate, and 80g of epimedium extract, passed through a 80-mesh sieve, qualified for future use .
[0079] 2. Mix the substances described in step 1 uniformly to obtain the pharmaceutical composition of the present invention.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com