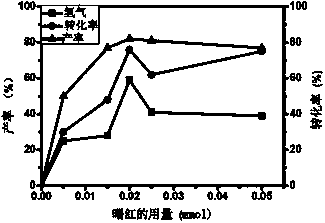
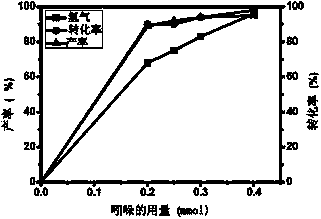
Visible light catalyzed cross-coupling hydrogen desorption method
A cross-coupling, visible light technology, used in organic chemistry methods, chemical instruments and methods, physical/chemical process catalysts, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of Graphene Oxide
[0047] (1) Expansion treatment of graphite
[0048] Heat 9mL of concentrated sulfuric acid to 80°C, add 1.6g of potassium peroxodisulfate and 1.6g of phosphorus pentoxide, stir at this temperature to completely dissolve the solid, then slowly add 2g of 320 mesh flake graphite, and finish adding within 5min , the mixture was reacted at 80°C for 4.5h, after the reaction was completed and cooled to room temperature, 350mL of deionized water was added, and after standing for 12h, the mixture was filtered through a 0.2μm filter membrane, and the residual acid and solids were washed with a large amount of water. Place at room temperature for 12h.
[0049] (2) Oxidation of expanded graphite
[0050] Take 80mL of concentrated sulfuric acid and place it in an ice bath at 0°C, add the expanded graphite obtained in step (1) into the sulfuric acid solution, then slowly add 10g of potassium permanganate under stirring, and ensure that the temperature ...
Embodiment 2
[0054] Synthesis of water-soluble graphene modified with sulfonic acid groups
[0055] Take 40mL of the graphene oxide solution obtained in 1, and use 5wt% Na 2 CO 3 Adjust the pH to 9-10. Add 160mg of sodium borohydride, react at 80°C for 1h, centrifuge after the reaction, wash the solid with water twice, then disperse in water to make a 0.5mg / mL dispersion, then add diazonium hydrochloride containing 0.095M p-sulfonic acid benzene 3.6 mL of salt, reacted in an ice bath for 2 hours, after the reaction was completed, 150 mL of acetone was added to the solution to precipitate a precipitate, which was centrifuged and washed three times with an acetone / water mixed solvent with a volume ratio of 3:1 to obtain The solid was dried in an oven at 50° C. and redispersed (ultrasonic power 150 W) into a 0.5 mg / mL aqueous solution.
Embodiment 3
[0057] Synthesis of water-soluble graphene-supported ruthenium dioxide nanoparticles modified with sulfonic acid groups
[0058] (1) Mix 30mL, 0.5mg / mL sulfonic acid-modified graphene aqueous solution with 80mL deionized water, stir, and then use ultrasonic treatment until clear (ultrasonic power is 150W) to obtain a uniform dispersion;
[0059] (2) Add 10mL, 0.0085mmol / L aqueous solution of ruthenium trichloride to the dispersion liquid, stir, and then obtain a uniform dispersion liquid through ultrasonic treatment (ultrasonic power is 150W);
[0060] (3) Add 10 mL, 0.082 mmol / L aqueous solution of trisodium citrate dihydrate to the dispersion liquid, and stir;
[0061] (4) Pass argon gas through the dispersion to remove oxygen, heat the dispersion system in an argon atmosphere, the temperature is controlled at 95°C, and the time is 10h;
[0062] (5) Centrifuge the reacted aqueous solution, wash 5 times with deionized water, centrifuge again, and finally wash with methanol, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com