Aryl bridged silsesquioxane monomer and preparation method thereof
A technology of sesquisiloxane and triethoxychlorosilane is applied in the field of aryl bridged silsesquioxane monomer and its preparation, which can solve the problems of high cost, difficulty in mass production, and reduced rigidity of bridge chain.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
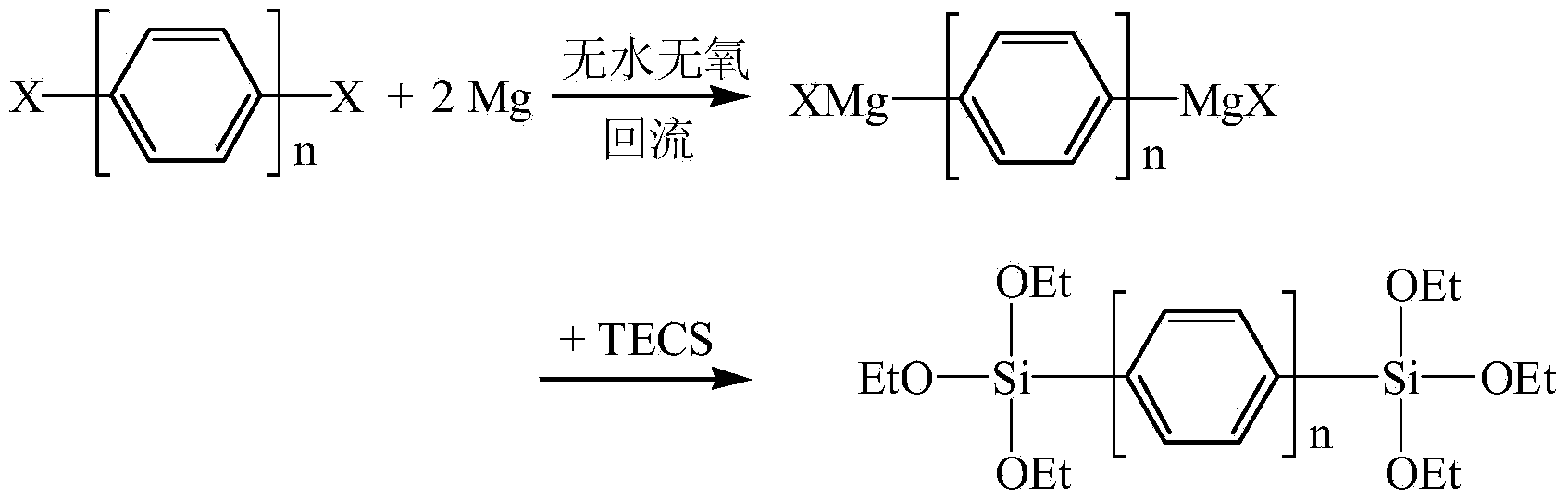
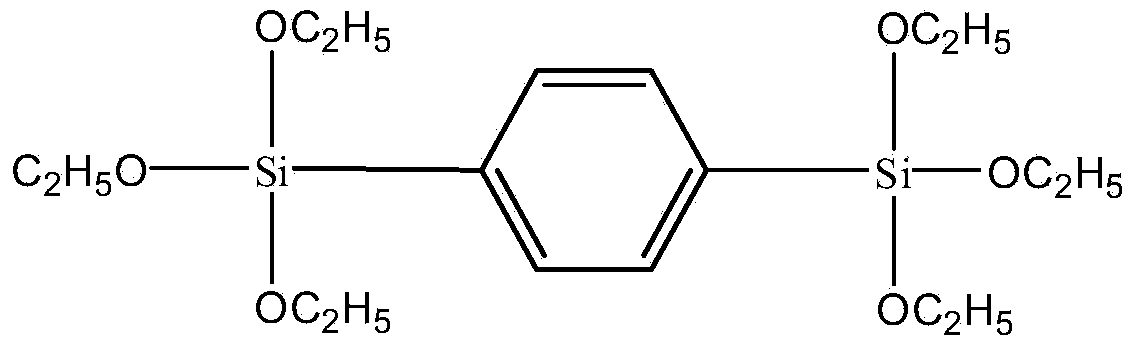
[0034] Embodiment 1: the preparation of 1,4-two (triethoxysilyl) benzene
[0035] Add 5g magnesium powder, 100mL TECS and two grains of iodine into the three-neck flask, vacuumize for 10min with double-pipe system, fill with nitrogen for 3min, and repeat this three times. Dissolve 16g of 1,4-dibromobenzene in 200mL of tetrahydrofuran, suck it up with a syringe, inject it dropwise into a sealed three-necked flask, protect it with nitrogen, stir it magnetically, heat it in an oil bath at 65°C, and reflux for 3 hours. When a gray-green suspension appeared, the reaction was stopped, cooled to room temperature, and tetrahydrofuran was distilled off under reduced pressure. Add anhydrous n-hexane under continuous stirring to precipitate magnesium and magnesium salts, and filter quickly to obtain a clear filtrate. Distill under reduced pressure to remove n-hexane and excess TECS in turn to obtain a brown oil. Raise the temperature and distill under reduced pressure to obtain a color...
Embodiment 2
[0038] Embodiment 2: the preparation of 1,4-two (triethoxysilyl) benzene
[0039] Add 8g of magnesium powder, 180mL of TECS and two grains of iodine into the three-neck flask, vacuumize for 15min with double-pipe system, and fill with nitrogen for 3min, and repeat this three times. Dissolve 22g of 1,4-dichlorobenzene in 400mL of dioxane, suck it up with a syringe, pour it dropwise into a sealed three-necked flask, protect it with nitrogen, stir it with a magnetic force, heat it in an oil bath at 100°C, and reflux it for 5 hours. When a gray-green suspension appeared, the reaction was stopped, cooled to room temperature, and tetrahydrofuran was distilled off under reduced pressure. Add anhydrous n-hexane under continuous stirring to precipitate magnesium and magnesium salts, and filter quickly to obtain a clear filtrate. Distill under reduced pressure to remove n-hexane and excess TECS in turn to obtain a brown oil. Raise the temperature and distill under reduced pressure to ...
Embodiment 3
[0042] Example 3: Preparation of 4,4'-bis(triethoxysilyl)biphenyl
[0043]Add 6g of magnesium powder, 100mL of TECS and two grains of iodine into the three-neck flask, vacuumize for 15min with double-pipe system, and fill with nitrogen for 5min, and repeat this three times. Dissolve 20g of 4,4′-dibromobiphenyl in 300mL of tetrahydrofuran, absorb it with a syringe, and inject it dropwise into a sealed three-necked flask, under nitrogen protection, magnetic stirring, heating in an oil bath at 80°C, and reflux for 5 hours. When a gray-green suspension appeared, the reaction was stopped, cooled to room temperature, and tetrahydrofuran was distilled off under reduced pressure. Add anhydrous n-hexane under continuous stirring to precipitate magnesium and magnesium salts, and filter quickly to obtain a clear filtrate. Distill under reduced pressure to remove n-hexane and excess TECS in turn to obtain a brown oil. Raise the temperature and distill under reduced pressure to obtain a ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com